TORCH Profile:- Part 4 – Toxoplasmosis (Toxoplasma gondii)
Toxoplasmosis (Toxoplamsma gondii)
What sample is needed for the TORCH profile?
- Venous blood is collected to prepare the serum.
- The other samples used are blood, urine, and spinal fluid for evidence of the infections for TORCH.
- Diagnosis can be confirmed by the culture of one of the specific pathogens or by increased levels of IgM against the specific pathogen.
What are the indications for TORCH?
- TORCH profile is done to find the cause of premature birth or abortion.
- TORCH screens infants for toxoplasmosis, cytomegalovirus, herpes simplex, rubella, and syphilis.
How will you describe the pathophysiology of TORCH?
- TORCH profile includes the following tests :
- Toxoplasmosis antibody.
- Rubella antibody.
- Herpes Simplex.
- Cytomegalovirus
- Some people include syphilis as well.
- These infections may lead to birth defects, growth delays, and problems in the baby’s brain and nervous system.
- If TORCH screening on infants is positive, more testing will be needed to confirm the diagnosis. The mother will also need to be checked.
- The test is ordered when a pregnant woman is suspected of having any of the TORCH infections.
- These infections can be serious during pregnancy because they can cross the placenta from the mother to the developing fetus and cause congenital defects in the newborn.
- The TORCH infections cause a syndrome characterized by:
- Microcephaly.
- Sensorineural deafness.
- Chorioretinitis.
- Hepatosplenomegaly.
- Thrombocytopenia.
- TORCH infection signs/symptoms are :
- Fever and poor feeding.
- The newborn is often small for gestational age.
- A petechial rash on the skin may be present, with small reddish or purplish spots due to bleeding from capillaries under the skin.
- An enlarged liver and spleen (hepatosplenomegaly) are common, and jaundice.
- Hearing impairment, eye problems, mental retardation, autism, and death can be caused by TORCH infections.
- The mother often has a mild infection with few or no symptoms.
- The examiner may test blood, urine, and spinal fluid for evidence of the infections for TORCH.
- Diagnosis can be confirmed by the culture of one of the specific pathogens or by increased levels of IgM against the pathogen.
Toxoplasmosis (Toxoplasma Gondii)
What sample is needed for the diagnosis of Toxoplasmosis?
- Blood to prepare the serum.
- Store the blood at 2 °C to 6 °C if the test is delayed for over 7 days.
- Serum should not be heat-inactivated because this may give false-positive results.
- This protozoan is a parasite of warm-blooded animals.
How will you define Toxoplasmosis?
- A protozoan parasite causes toxoplasmosis (Toxoplasma Gondii).
- Protozoan parasites are unicellular and eukaryotic.
How will you describe the epidemiology of Toxoplasmosis?
- The antibody to T.gondii varies between populations. It ranges from 96% in Western Europe to 10% to 40% in the United States of America.
- The patients with AIDs are seropositive for T. gondii, and roughly 25% to 50% will develop encephalitis.
- This is a protozoan that is present in all warm-blood animals.
- Toxoplasma gondii was first discovered in North African rodents and has been observed in numerous birds and mammals worldwide, including humans.
- The cat is the definitive host.
- Toxoplasma gondii is the most common causative agent of toxoplasmosis.
- This is found worldwide because so many animals harbor it.
- 15% to 20% of the American population has this infection.
- The highest record, 93%, is found in Parisian females who eat undercooked or raw meat, and 50% of cases are seen in their children.
- A number of babies are infected through the transplacental route.
- This is also seen in the USA due to undercooked meat.
- Oocyst is hardy and can survive for a longer period.
- These organisms have no flagella.
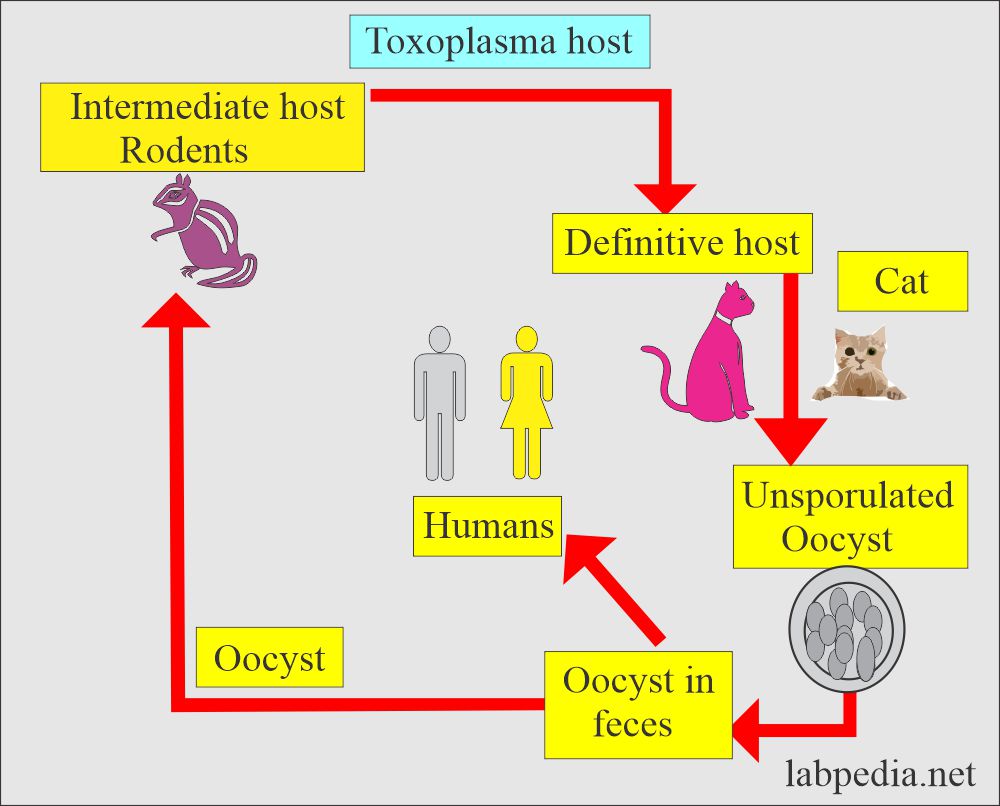
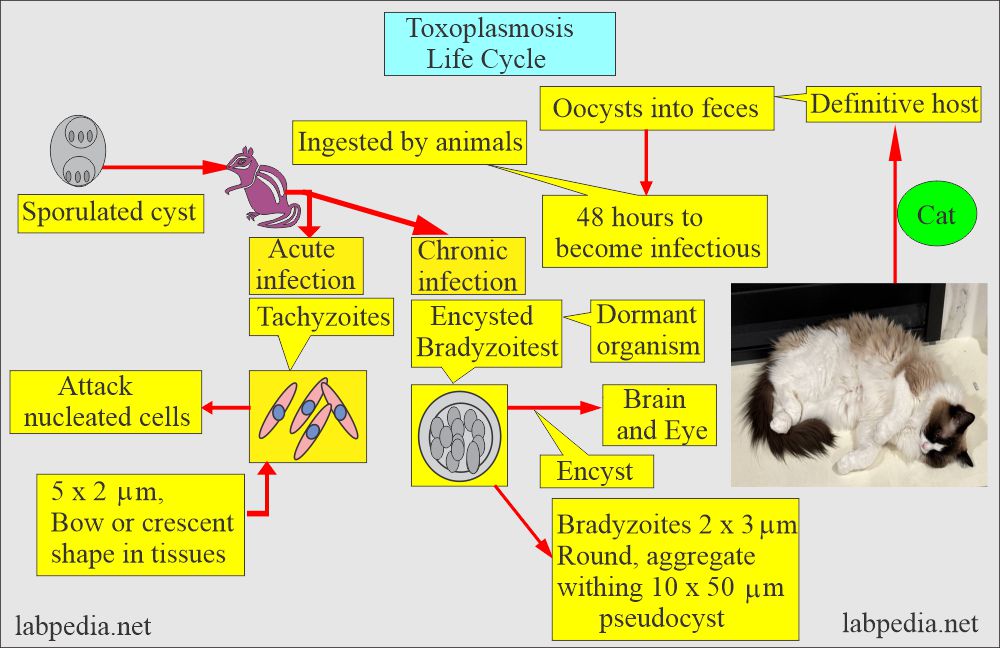
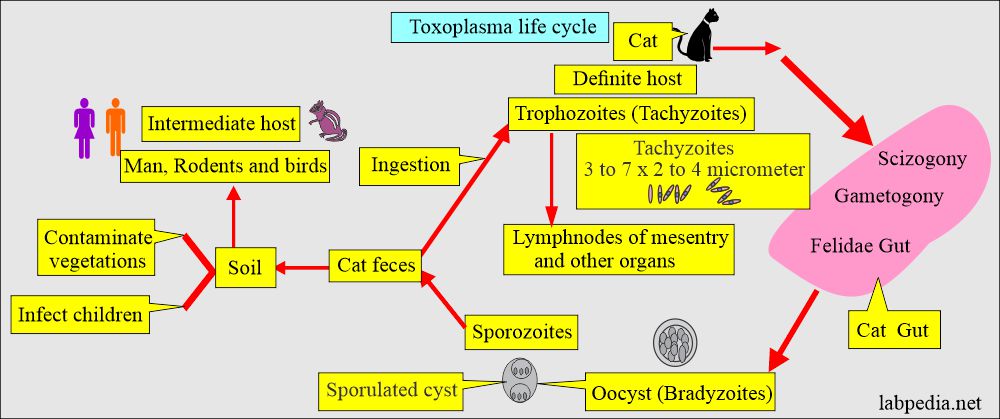
How will you discuss the life cycle of Toxoplasma Gondii?
- Toxoplasma has a complete life cycle as coccidian in the filedae (carnivorous animals including cats and big cats).
- The definitive host:
- These are the house cats. Cats are the only definitive host.
- Domestic cats are the source of the disease because the oocysts are often present in their feces.
- Oocysts can survive in the soil for over a year.
- It can lead to infecting humans from the cat litter box that contains oocysts in the stool.
- In the cat, the parasite develops a sexual cycle, and oocysts are eventually excreted in the feces.
- Trophozoites are crescent-shaped and can spread in the cat’s organs and tissues.
- Later on, these develop into cysts.
- The intermediate host:
- The intermediate host is a rat and a man.
- After ingestion, the parasite, an obligate intracellular organism, spreads widely via blood circulation.
- The ingested oocyst will encyst various organs like muscles and remain dormant for many years or the host’s life.
- Cysts may form in the brain, muscles, and eye.
What is the source of the spread of Toxoplasmosis Gondii?
- Humans may acquire the disease by ingesting uncooked meat and contaminated material.
- Mode of transmission:
- There may be fecal contamination.
- Food.
- Water.
- Soiled hands.
- Inadequate cooked or infected meat.
- Raw milk.
- Blood transfusion transmission of toxoplasmosis has been recently recognized, particularly with white blood cell concentrate.
- Patients at risk are those receiving immunosuppressive agents or corticosteroids.
- Exposure to feces of cats or other infected material.
- Toxoplasma Gondii spread due to:
- Hand-to-mouth contamination of infected oocyst in cat feces.
- Ingesting contaminated meat.
- Transplacental spread during delivery.
- Transplacental transmission usually takes place in the course of an acute or undiagnosed maternal infection.
- The expected incidence of congenital toxoplasmosis is 2.7 per 1000 live births.
What is the disease pattern of Toxoplasmosis?
- After ingesting the cysts, this protozoan is an obligatory intracellular parasite.
- This will spread through the blood, and cysts may form in the brain and muscles.
- These cysts may be seen in the eye.
- A congenital infection that shows:
- Hydrocephalus or microcephaly.
- Encephalomyelitis.
- Chorioretinitis.
- Cerebral calcification.
- There are lesions in the viscera.
- There may be acute enlargement of the lymph nodes.
- Severe illness leads to Myocarditis, Pneumonitis, Hepatitis, Meningoencephalitis, and ocular lesions.
- Types of toxoplasmosis are:
- Congenital toxoplasmosis.
- Cerebral toxoplasmosis.
- Toxoplasmosis in the immunocompromised patients.
- Cerebrospinal fluid is abnormal in 2/3 of the cases, showing positive xanthochromia and raised protein levels.
What are the signs and symptoms of Toxoplasmosis?
Primary infection:
- Many patients may remain asymptomatic, especially children.
- Later on, this results in generalized infection:
- The patient may have fatigue, and the initial presentation is an enlargement of the lymph nodes,
- Patients may have chills and fever.
- The Patient may have a headache and myalgia.
- Chronic cases may develop a maculopapular rash.
- Severe symptoms may be seen in patients with encephalomyelitis, myocarditis, or hepatitis.
- Spontaneous recovery follows acute febrile disease; the organism can localize and multiply in any organ or circulatory system.
Acquired infection:
- This is frequently mild.
- Chills, fever, headache, enlarged lymph nodes, and extreme fatigue exist.
- A chronic form of toxoplasmic lymphadenopathy may exist.
- Reactivation of cerebral toxoplasmosis may be seen in AIDS patients.
- Encephalitis is seen in AIDS patients. In these patients, CD4+ cell numbers fall below 100 x 109/L.
Congenital infection:
- The Toxoplasma parasite is transferred to the fetus through the placenta, where the mother acquires active infection near conception or during the pregnancy.
- Mothers, during the first trimester, infect 14% of the fetuses.
- During the second trimester, 29% of the fetuses are involved.
- It reaches 59% of the fetuses during the third trimester (another reference is 90% in the late third trimester).
- About 90% of the ladies acutely infected are asymptomatic.
- Unlike toxoplasmosis in later life, it is very severe in these patients.
- It results in central nervous system malformation.
- There may be prenatal mortality.
- Typically, these patients present with the following:
- Cerebral calcificationion.
- Chorioretinitis.
- Hydrocephalus or microcephaly.
- Infants who are serologically positive at birth may fail to display the following:
- Neurological abnormalities.
- Ophthalmic abnormalities.
- Generalized illness at birth.
- 75% of the cases of a congenitally infected newborn are not seropositive or not diagnosed at birth:
- The disease remains dormant.
- Or discover when the patients will develop:
- Chorioretinitis.
- Unilateral blindness.
- Neurological abnormalities.
- Congenitally infected babies also have general S/S like:
- Fever.
- Rashes.
- Jaundice.
- Hepatosplenomegaly.
- Acquired infection during pregnancy (in-utero) may lead to abortion or stillbirth.
What are the Late complications of Toxoplasmosis?
-
- Chorioretinitis.
- There may be mental retardation.
- Palsy.
- Deafness.
What are the serological findings of congenital toxoplasmosis?
- A raised level of IgM supports the congenital infection. There are other conditions for giving IgM responses.
- So, raised levels of IFA-IgG support the diagnosis of toxoplasmosis.
- The rising titer of IgG is important to confirm the diagnosis and avoid maternal IgG antibodies.
- Nonspecific changes are raised in the WBC count, eosinophils, and platelet count.
- Increased serum total IgM, GGTP, and LDH.
- Can get chorionic villi culture in the first trimester.
- Fetal cord blood and amniotic fluid at 2 to 22 weeks of gestation.
- PCR of amniotic fluid.
What are the complications of congenital toxoplasmosis?
- Hydrocephalus.
- Microcephaly.
- Chronic retinitis.
- Convulsion.
How to prevent congenital toxoplasmosis?
- Avoid touching the mucous membrane of the mouth and eye while handling raw meat.
- Wash hands thoroughly after handling raw meat.
- Cook the meat at >66 °C.
- Wash the kitchen surfaces that come in contact with the raw meat.
- Wash the fruits and vegetables thoroughly before eating.
- Prevent access to flies, cockroaches, other insects, and vegetables and fruits.
- Avoid contact or wear gloves when handling cat feces-contaminated materials.
What is the interpretation of the serological tests in pregnant women?
| IgG antibody | IgM antibody | Interpretations of the serological test |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
How will you diagnose Toxoplasmosis?
The culture:
- The culture of Toxoplasmosis gondii is often very difficult, so the serology supports the diagnosis.
Biopsy of the lymph nodes:
- Lymph node biopsy is suggestive by the small histiocyte groups involving the germinal centers.
- This pattern is not specific for the confirmation of toxoplasmosis.
- The presence of the organism is rarely seen in the lymph node.
Serology of Toxoplasmosis:
- Serologic tests make the backbone of the diagnosis of toxoplasmosis.
- The enzyme-linked immunofluorescent assay (EIA) is the method of choice for detecting IgM, indicating acute infection.
- The various methods or techniques used for T. gondii antibody are:
- Enzyme-linked immunoassay (EIA).
- Indirect hemagglutination (IHA).
- Indirect fluorescent antibody (IFA).
- Sabin-Feldman dye test. It is a methylene blue dye test.
- This dye test requires living toxoplasma organisms.
- Complement fixation test.
-
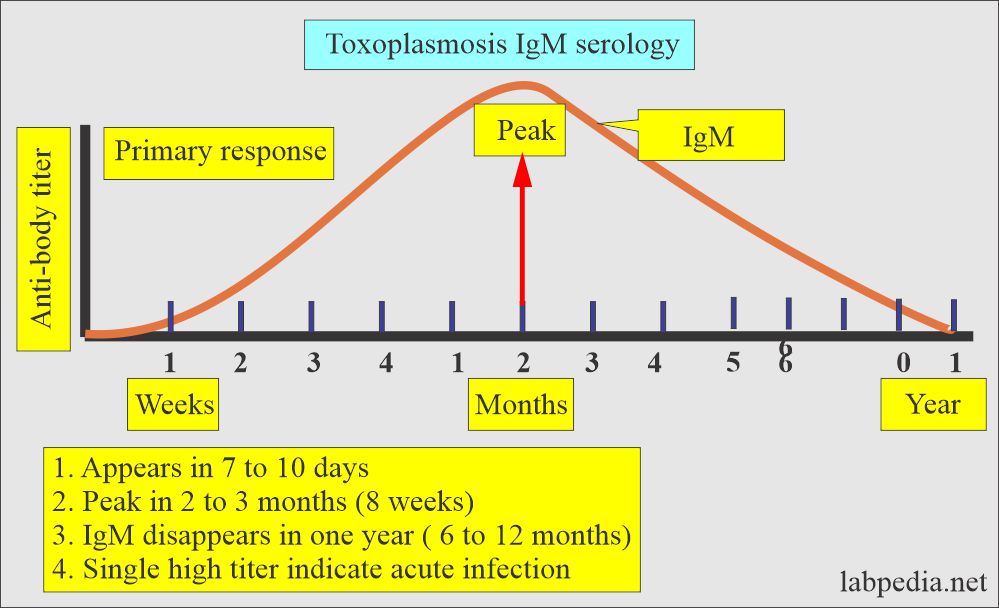
IgM:
- It starts rising about 7 to 10 days after the infection.
- The peak level reaches around 8 weeks (the range is 4 to 10 weeks).
- It becomes nondetectable by 6 to 12 months after infection.
- IgM antibody is needed to confirm the present infection.
- Antibody IgM titer <1:16 = shows no exposure to the virus.
- Antibody IgM titer >1:4 to 1:256 = acquired infection in last 18 months.
- Antibody IgM >1: 1024 = Acquired infection in last 4 months.
-
IgG:
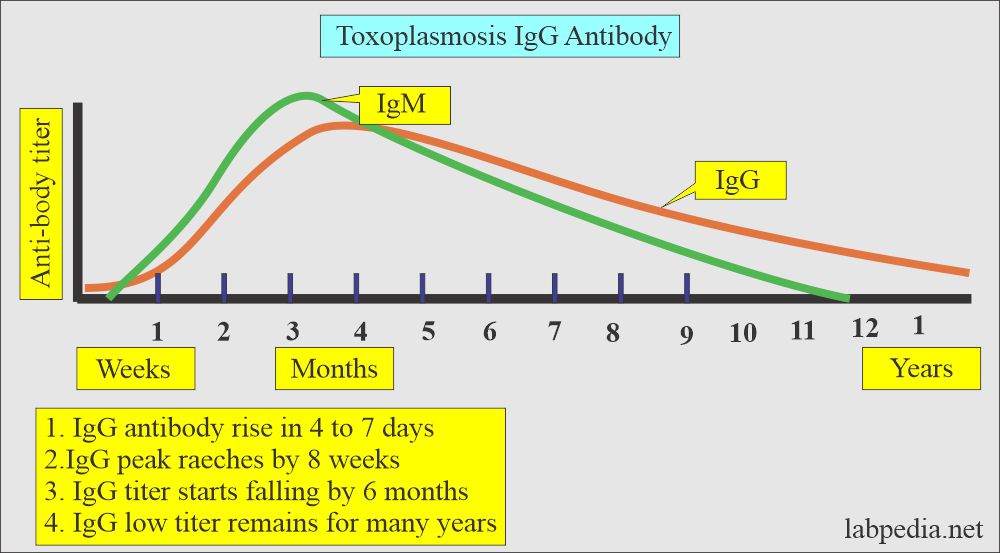
- IgG antibody starts rising about 4 to 7 days after the infection.
- IgG reaches peak level in about 8 weeks (range is 4 to 10 weeks).
- It starts falling slowly by 6 months.
- While low titers persist for many years.
- The anti-nuclear antibody may produce a false positive result.
- IgG antibody titer represents present or past infection.
- The rise in the IgG or IgM antibody to toxoplasma is rapid.
- There is a significant rise by the end of the first week.
- ELISA and agglutination kits are commercially available.
How will you interpret the Toxoplasmosis antibody?
| IgG antibody | IgM antibody | Interpretations |
|
|
|
|
|
|
|
|
|
|
|
|
How will you treat Toxoplasmosis?
- Most infections are self-limiting.
- Treatment of choice combines Trisulfapyrimidines and Pyrimethamines (Daraprim) needed for chorioretinitis.
- Corticosteroids may be helpful.
Questions and answers:
Question 1: What are best tests for the diagnosis of toxoplasmosis?
Question 2: What is the significance of IgM for the diagnosis of toxoplasmosis?