Serum Proteins (Total Serum Proteins)
Serum Proteins
What sample is needed for Serum Protein?
- This is done on the patient’s serum.
- Analyze a fresh sample or store it at 4 °C for >72 hours.
- For 6 months, stored at -20 °C.
What precautions are needed for Serum Proteins?
- Avoid prolonged application of a tourniquet. This will lead to hemoconcentration and give a false rise in values.
- Avoid hemolysis and lipemic serum.
- Avoid blood from the side of the I/V infusion, which will lower the result.
- Drugs like anabolic steroids, androgens, dextran, growth hormone, progesterone, and insulin increase the protein level.
- Some of the drugs decrease the level, like estrogen, hepatotoxic drugs, and oral contraceptives.
What are the indications for Serum Proteins?
- This is the best marker for liver function activity.
- This test will assess renal function.
- Assess the protein-losing diseases of the intestines and kidneys.
- Assess the immune disorder.
- Assess impaired nutrition.
- To evaluate the chronic edematous conditions.
- To evaluate patients with malignancies like lymphoma and myeloma.
What are the types of Serum Proteins?
- Human bodies contain thousands of proteins. These are present in the intracellular and extracellular spaces.
- These are present in the blood, urine, CSF, amniotic fluid, saliva, feces, and peritoneal and pleural fluids.
- Proteins are divided into:
- Fibrous, e.g., fibrinogen, troponin, collagen, and myosin.
- Globular, e.g., hemoglobin, enzymes, peptide hormones, and plasma proteins.
- Globular proteins are compact and have little or no water space in the molecule’s interior.
- Most globular proteins retain their biological activities within the narrow range of pH and temperature.
- If these are exposed to a high temperature, their molecule is denatured.
- Conjugated proteins examples are lipoproteins, glycoproteins, mucoproteins, metalloproteins, mucoproteins, and phosphoproteins.
What are the properties of serum proteins?
- Molecular size influences the properties of various proteins. The smaller molecules can be separated by dialysis, ultrafiltration, chromatography, and density gradient ultracentrifugation.
- Proteins’ electrical charges lead to their mobility in the electrical field. These proteins are separated by electrophoresis.
- The difference in the solubility of the proteins depends upon the solvents’ pH, temperature, ionic strength, and dielectric constant.
- Specific binding to the antibodies, hormone receptor, and coenzymes. This is their unique property, and these proteins can bind to specific antibodies, which is the basis of the immunochemical assay.
- Proteins are the source of nutrition and a buffer system.
- Proteins are part of muscles, hormones, enzymes, hemoglobin, and transport protein.
What are the various forms of proteins?
- Total serum proteins consist of:
- Prealbumin.
- Albumin.
- Globulins.
- Other proteins included are:
- Complements.
- Fibrinogen.
- C – Reactive protein.
- Miscellaneous proteins are :
- Myoglobin.
- Troponin.
- Fibronectin.
- Amyloid.
- Proteins found in other body fluids are:
- Urinary protein.
- Cerebrospinal fluid protein.
- Protein in the ascetic and pleural fluid.
What are the functions of proteins?
- Their main function is maintaining the osmotic pressure, which keeps the fluid within vascular spaces.
- Albumin is made in the liver, 60% of the total proteins.
- Albumin’s main function is to maintain the colloid osmotic pressure.
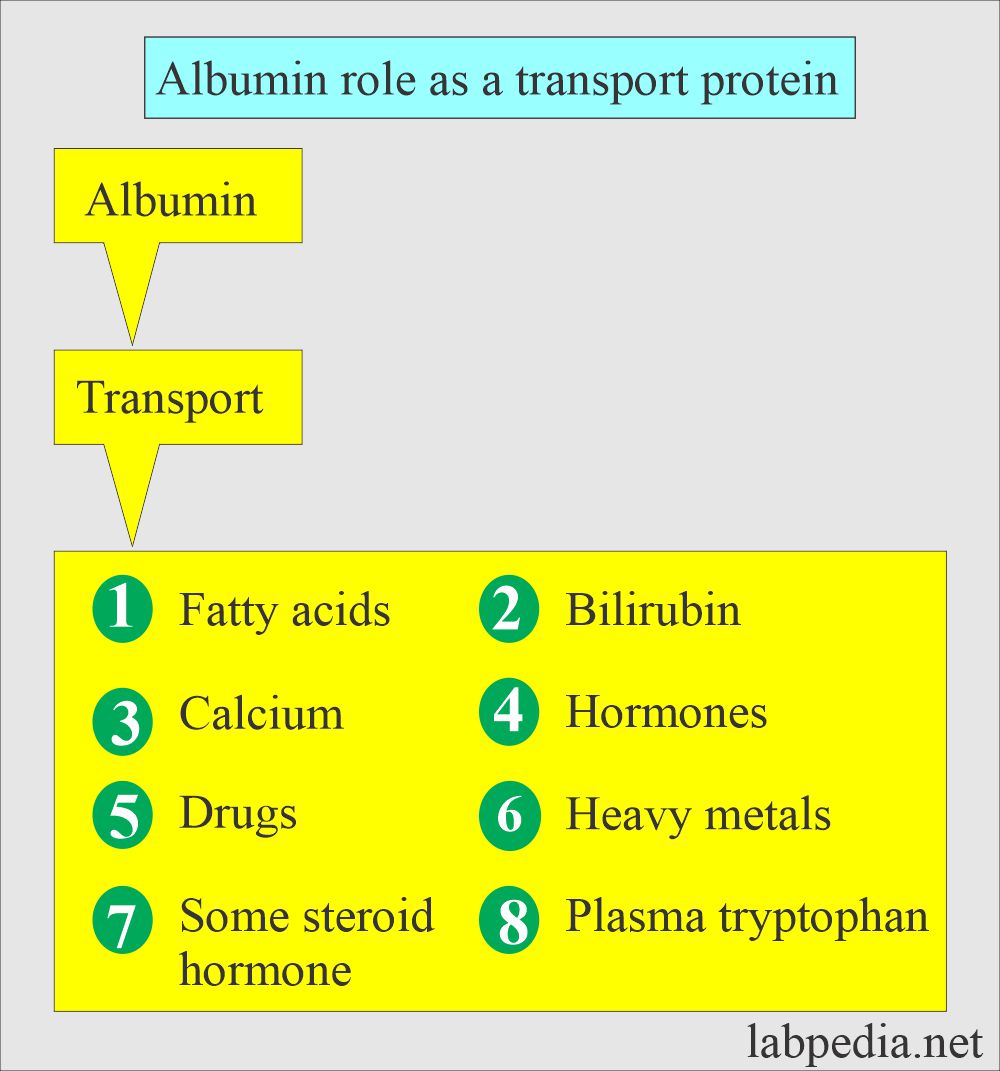
- Albumin acts as a transport protein for drugs, hormones, and enzymes.
- Albumin is synthesized in the liver, so it measures liver function.
- Albumin’s half-life is 12 to 18 days, so liver damage will not be seen during this period.
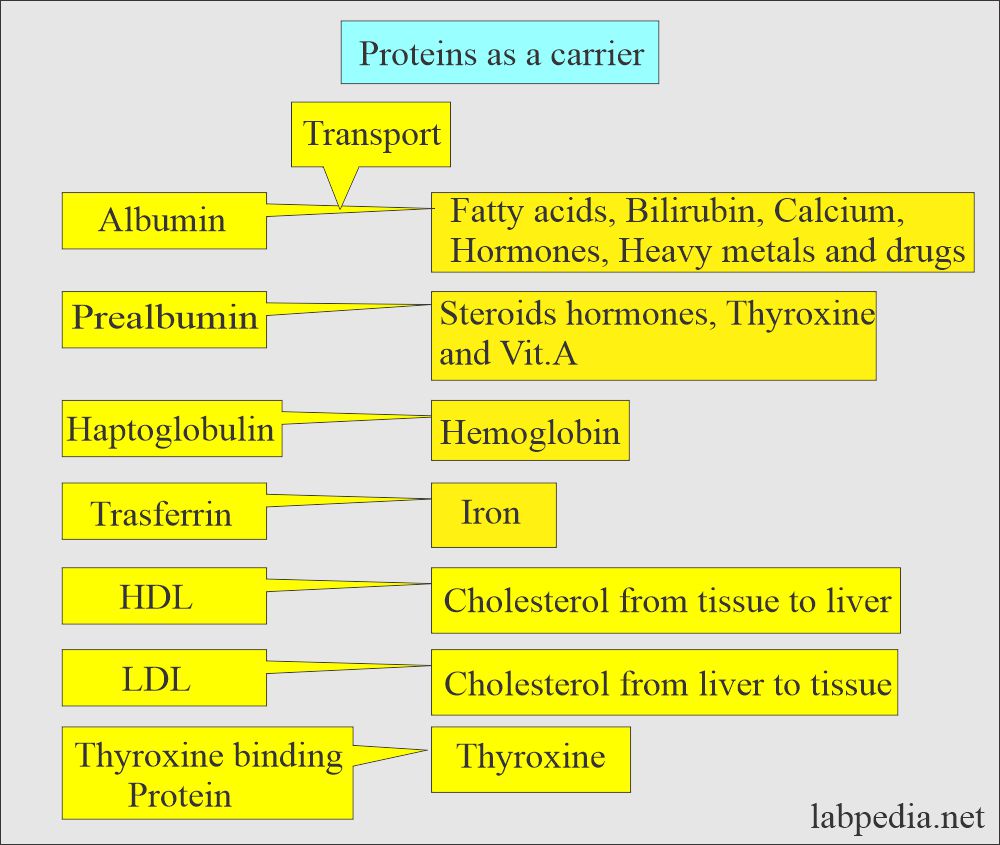
- Proteins act as carrier proteins, and some of these are:
- Haptoglobin.
- Prealbumin.
- Transferrin.
- Proteins demonstrate various biological functions:
- Enzymes catalyze the biochemical reaction, which is essential for metabolism.
- Proteins, polypeptides, and oligopeptide hormones regulate metabolism.
- Antibody proteins and the complement system protect against infection.
- Proteins maintain the osmotic pressure of the plasma.
- Hemoglobin carries oxygen.
- Protein coagulation factors take part in hemostasis.
- They transport hormones, vitamins, metals, and drugs.
- Proteins act as carrier proteins for transporting other proteins, hormones, and drugs. These are separated on the electrophoresis.
What are the types of Proteins and their functions?
| Type of protein | Quantity | Site of formation | Functions |
| Albumin | 60% | Liver | Maintain blood osmotic pressure |
| Globulins | 36% | ||
| Alpha Globulin | Liver |
|
|
| Beta Globulins | Liver |
|
|
| Gamma Globulins | Lymphatic system | Take part in the immune system | |
| Fibrinogen | 4% | Liver | Take part in blood coagulation |
What is the role of Albumin as a carrier protein?
- Transport protein.
- Maintain osmotic pressure.
- Source of endogenous amino acid.
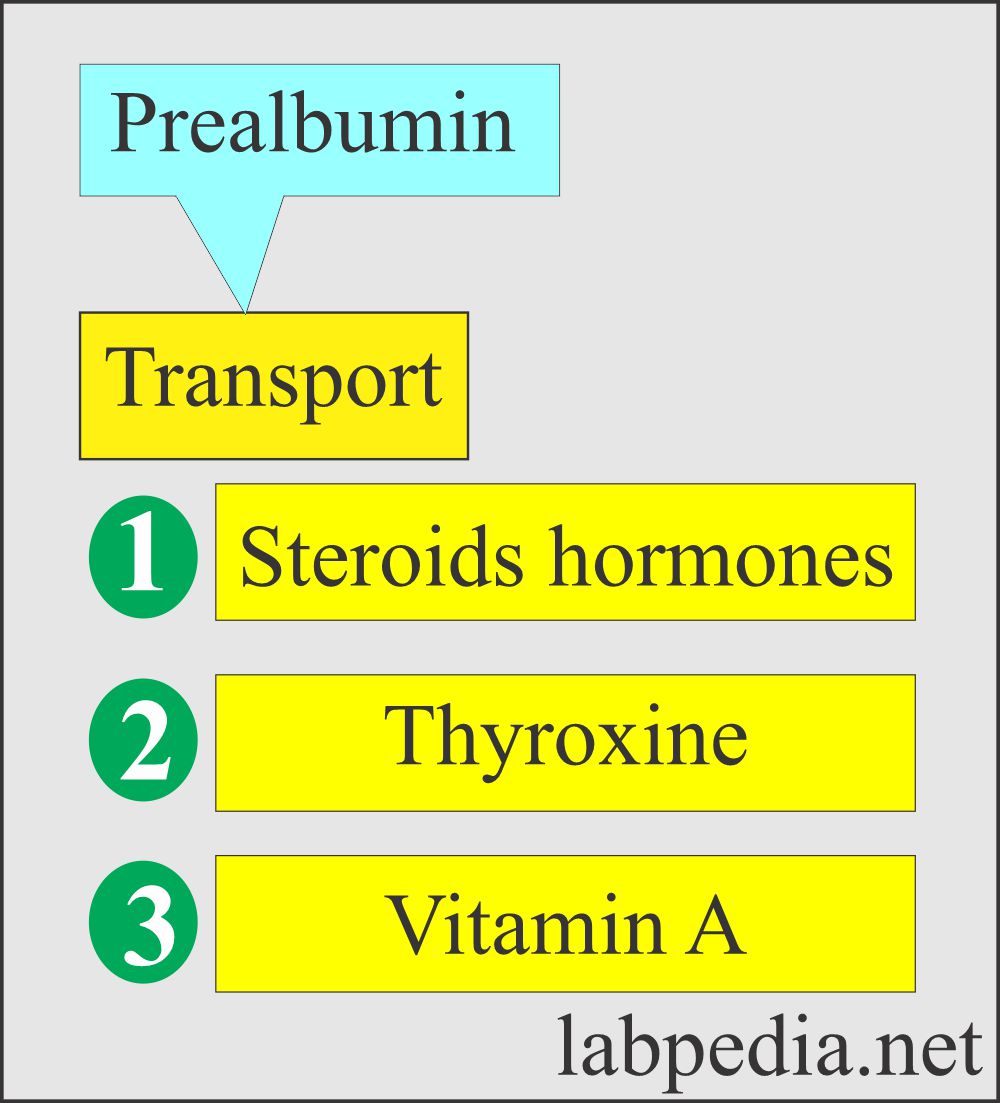
What is the role of Prealbumin as a carrier protein?
- Transport protein for T3 and T4, steroid hormones, and Vit. A.
What is the normal Serum proteins?
Source 1
| Total Proteins | |
| Age | g/dL |
| Cord blood | 4.8 to 8.0 |
| Premature | 3.6 to 6.0 |
| Newborn | 4.6 to 7.0 |
| one week | 4.4 to 7.6 |
| 7 months to one year | 5.1 to 7.3 |
| 1 to 2 years | 5.6 to 7.5 |
| ≥ 3 years | 6.0 to 8.0 |
| Adult | |
| Ambulatory | 6.4 to 8.3 |
| Recumbent: | 6.0 to 7.8 |
| >60 years | Lower by ∼0.2 |
| ALBUMIN | |
| 0 to 4 days | 2.8 to 4.4 |
| 4 days to 14 days | 3.8 to 5.4 |
| 14 to 18 years | 3.2 to 4.5 |
| 18 to 60 years | 3.4 to 4.8 |
| 60 to 90 years | 3.2 to 4.6 |
| >90 years | 2.9 to 4.5 |
- To convert into SI unit x 10 = g/L
Source 4
- Total proteins
- Adult = 6 to 8.0 g/dL.
- Child newborn = 4.6 to 7.4 g/dL.
- Child 1 to 3 years = 5.9 to 7.0 g/dL.
- Child 4 to 6 years = 5.9 to 7.8 g/dL.
- Albumin
- Adult = 3.5 to 5 g/dL.
- Premature infant = 3 to 4.2 g/dL
- Newborn = 3.5 to 5.4 g/dL
- Infants = 4.4 to 5.4 g/dL
- Child = 4 to 5.9 g/dL
What are the causes of hyperproteinemia?
- Dehydration
- Monoclonal gammopathy
- polyclonal gammopathy
What are the causes of Hypoproteinemia?
- Decrease protein synthesis like liver diseases and decreased amino acid intake
- Increased protein loss like nephrotic syndrome
- Increased protein catabolism, like in malignancies and inflammation
What are the causes of Hyperalbuminemia?
- This may be due to dehydration.
What are the causes of hypoalbuminemia?
- Decreased albumin synthesis is seen in liver diseases and decreased amino acid intake.
- Increased albumin loss is seen in kidney diseases like nephrotic syndrome, blood loss, and burns.
- Increased catabolism of albumin seen in malignancy and inflammation.
Questions and answers:
Question 1: What is the role of prealbumin?
Question 2: What are the types of proteins?
- Please see more details on protein serum electrophoresis.