Phosphorus (P), Inorganic Phosphate (PO4), Inorganic Phosphorus
Phosphorus (P)
What sample is needed for Phosphorus (P)?
- This test is done in the patient’s serum.
- The heparinized plasma can be used.
- Separate the serum from the blood as soon as possible, maximum within one hour.
- The fasting serum is preferred.
- The separated serum is stable at 4 °C for several days.
- The frozen sample is stable for several months.
What are the Precautions for Phosphorus (P)?
- Avoid venous stasis.
- Hemolysis, icteric serum, and fluoride interfere with the chemical reaction.
- Be Careful about the phosphorus contamination of glassware.
- There is a diurnal variation with an increased level in the PM sample. So, a fasting (AM) sample is preferred.
- Exercise leads to an increase in level.
- Avoid anticoagulants like oxalate, citrate, and EDTA.
- The phosphate level in serum increases if the sample is left at 37 °C at room temperature for a long time.
What are the Indications for Phosphorus (P)?
- This will give an idea of renal and bone diseases.
- This test is done to investigate calcium abnormality.
- This test is done to evaluate parathyroid abnormality.
How will you discuss the pathophysiology of Phosphorus (P)?
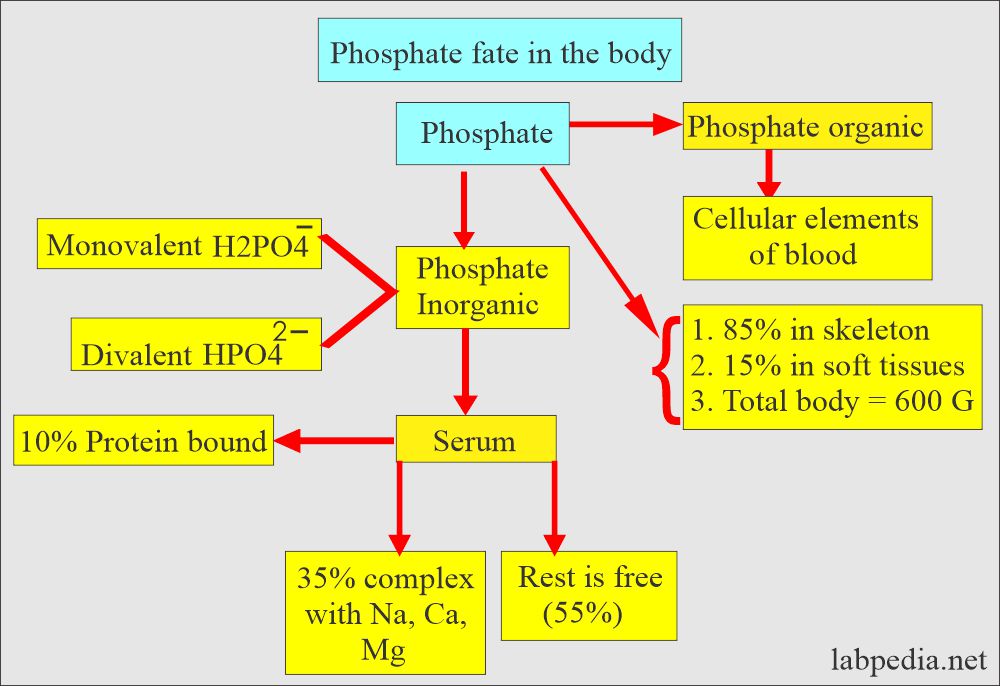
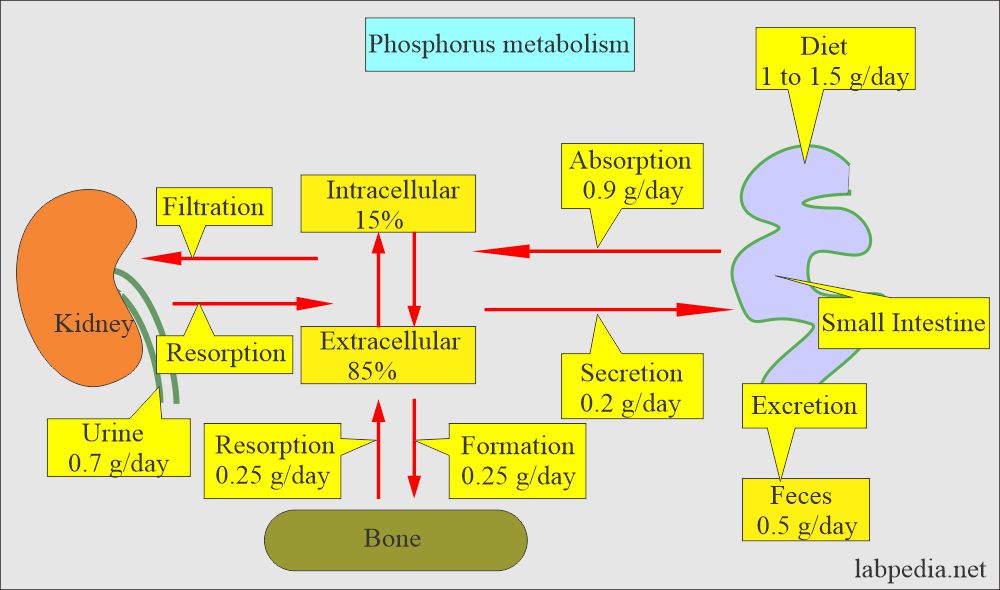
- Phosphates are usually measured as phosphorus ions.
- Most of the phosphorus in the body is in phosphate, so these are used interchangeably. So, it exists in the body:
- Inorganic phosphate.
- Organic phosphate esters.
- Most of the phosphorus is in organic form, and a very small amount is in inorganic form (2.5 to 4.5 mg/dL).
- So, we measure inorganic phosphate when there is a request for phosphorus, phosphate, or inorganic phosphate.
- The organic phosphate esters which are not measured are part of or present in the following:
- Synthesis of phospholipids in the cell membranes (present within cells).
- Associated with nucleoproteins.
- Hexoses (glucose-6-phosphate).
- Deoxygenated hemoglobin in the RBCs.
- ATP (adenosine triphosphate) is an energy source in metabolism.
- The energy source for enzymes like 2,3 diphosphoglycerate.
- There is a diurnal rhythm, with higher values in the afternoon and evening, which may be double those found in the morning sample.
What is the distribution of the Phosphorus (P) and phosphate?
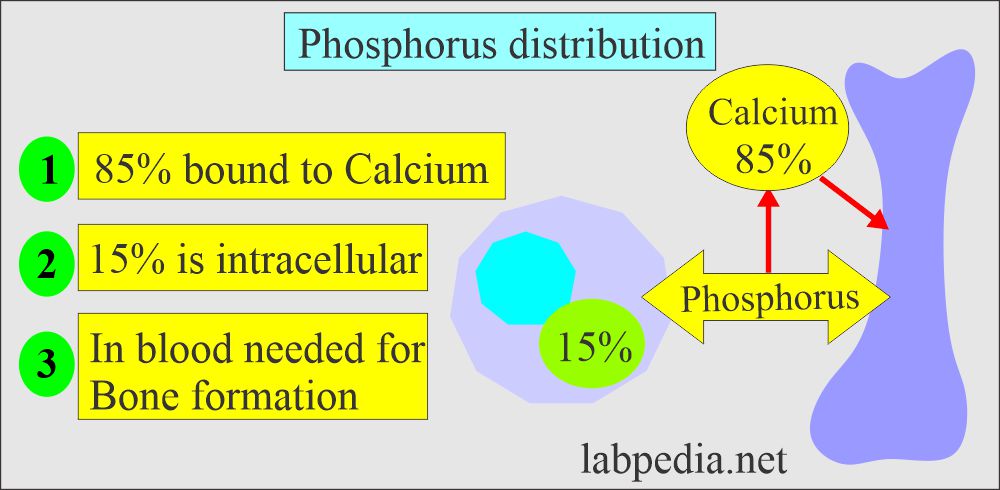
- In our body, 85 % of the phosphorus is combined with Calcium in the bone.
- The remaining 15 % is in the cells.
- 10% (10% to 15%) of phosphate in serum is protein-bound.
- 35% of serum is complexed with sodium, magnesium, and calcium.
- Inorganic phosphate ions (H2PO4¯, HPO4¯ ¯ ) are mostly confined to the extracellular fluid. Their main role is a buffer system.
- 80% of inorganic phosphate at pH 7.4 is in the form of HPO4¯ ¯.
- The rest is free in the serum.
- The following table shows the relative distribution of phosphate and Calcium in the body.
| Site | Phosphate | Calcium |
|
85% | 99% |
|
<0.1 % | <0.2% |
|
15% | 1% |
|
600 | 1000 |
- Most of the phosphorus in the blood exists as phosphate.
- Phosphate in blood exists in two forms:
- Monovalent Phosphate (H2 PO4)¯.
- Divalent Phosphate (HPO4)2¯.
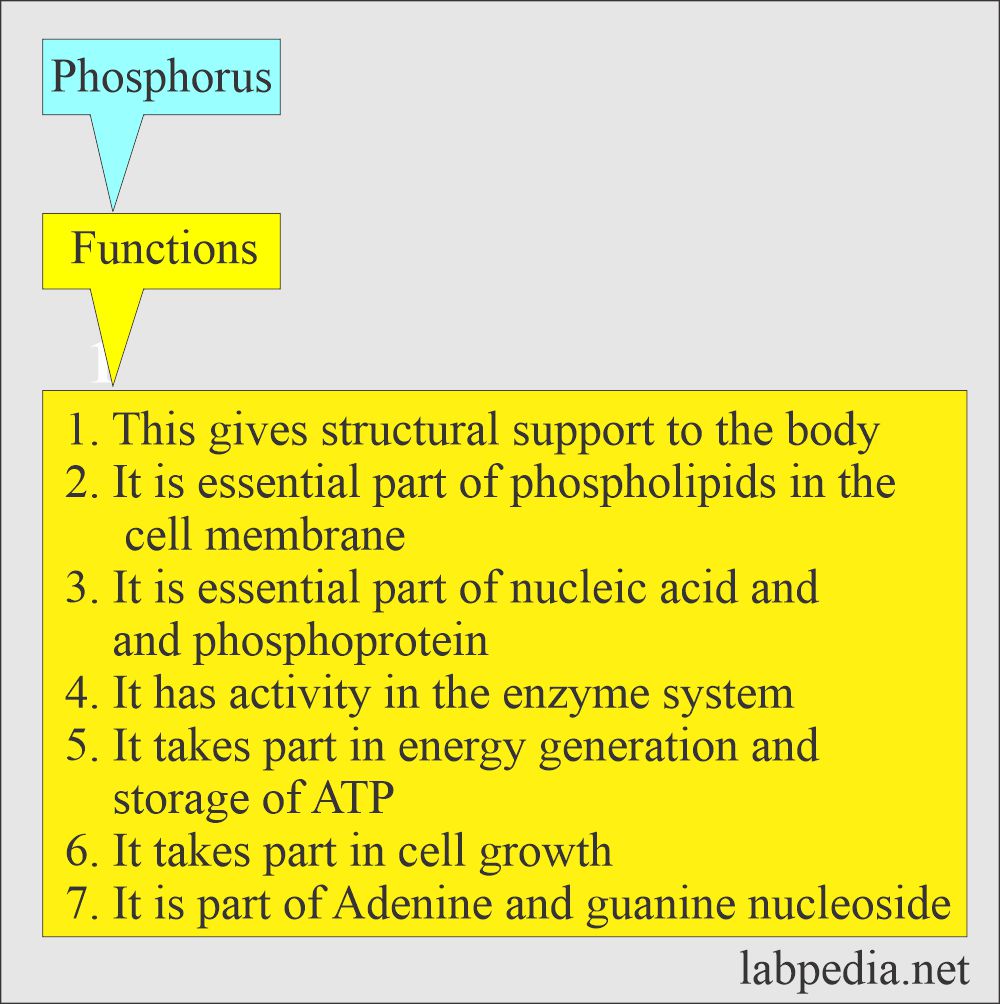
What are the functions of Phosphorus (Phosphate)?
- Phosphate is required for:
- Formation of the bone:
- In the metabolism of glucose and lipids.
- In the maintenance of acid-base balance.
- It is needed to store and transfer energy from one site to another.
How will Phosphorus (Phosphate) absorption be?
- Phosphorus enters the RBC with glucose, so its level is lowered after ingesting carbohydrates.
- The dietary absorption of phosphate is very efficient; there is rarely a phosphate deficiency.
- Malabsorption and antacids can decrease the absorption in the GI tract.
- The renal excretion maintains the balance of phosphorus in the dietary intake.
- Phosphate level varies during the day:
- Low values around 10 AM.
- High values after 12 hours later.
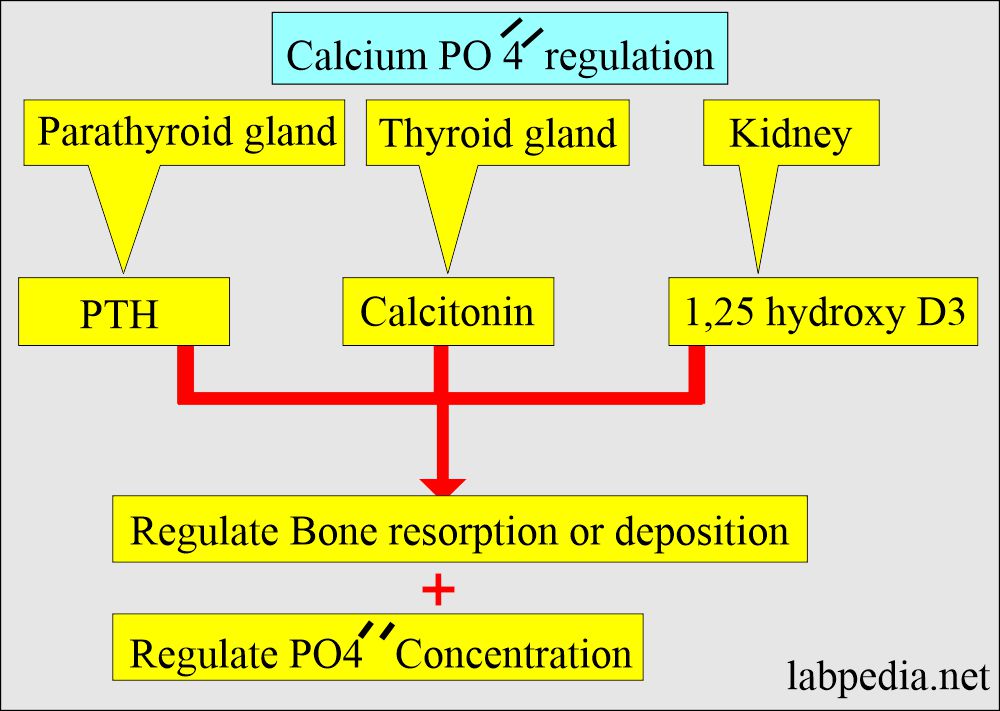
- Phosphorus level is dependent upon the following:
- Calcium metabolism.
- Parathyroid hormone PTH.
- Renal excretion.
- Intestinal absorption.
- PTH tends to decrease phosphate reabsorption in the kidney.
- PTH and Vit. D stimulates the absorption of phosphate from the intestinal.
- When calcium levels are decreased, then the phosphorus level increases.
- When the calcium level is increased, then the phosphorus level is decreased.
- The kidney maintains this inverse ratio by increasing the excretion. The principal route of excretion is urine.
What is the normal level of Phosphorus (P)?
Source 1
| Age | mg/dL |
|
3.7 to 8.1 |
|
5.4 to 10.9 |
|
4.5 to 9.0 |
|
4.5 to 5.5 |
|
2.7 to 4.5 |
| >60 year | |
|
2.3 to 3.7 |
|
2.8 to 4.1 |
| Urine 24 hours | |
|
<1.0 g/day |
|
0.4 to 1.31 |
- The constant daily diet contains 0.9 to 1.5 g of Phosphorus and 10 mg calcium/ kg.
- To convert into SI unit x 0.323 = mmol/L
Source 2
- Adult = 3 to 4.5 mg/dL (0.81 to 1.45 mmol/L).
- Child = 4.4 to 6.5 mg/dL (1.29 to 2.26 mmol/L).
- Newborn = 4.3 to 9.3 mg/dL (1.43 to 3 mmol/L)
- Urine (on a non-restricted diet) = 0.4 to 1.3 g/day (12.9 to 42.0 mmol/day).
- These values may vary from different sources.
What are the causes of increased Phosphorus (P) or hyperphosphatemia?
The level is more than 4.7 mg/dL:
- Renal diseases with increased blood urea ( BUN) and creatinine.
- Hypoparathyroidism with raised phosphate and decreased calcium. However, the renal function will be normal.
- Hypocalcemia.
- Excessive intake of Vit.D.
- Milk-alkali syndrome.
- Bone tumors and metastases.
- Liver diseases and cirrhosis.
- Addison’s disease.
- Acromegaly.
- Increased dietary intake.
- Sarcoidosis.
- Acidosis.
- Hemolytic anemia.
What are the causes of decreased levels of phosphorus (P) or hypophosphatemia?
The level is less than 2.4 mg/dL:
- Decreased intestinal absorption.
- Increased renal excretion
- Hyperparathyroidism.
- Hyperinsulinemia.
- Rickets ( Vit.D deficiency ).
- Diabetic coma.
- Vomiting and severe diarrhea.
- Liver diseases.
- Acute alcoholism.
- Severe malnutrition and malabsorption.
- Hypercalcemia due to any cause.
- Gram-negative septicemia.
- Chronic intake of antacids.
- Alkalosis.
- Causes according to the mechanism of Hyperphosphatemia:
- Increased renal reabsorption:
- Excess vit.D
- Hypogonadism
- Hypoparathyroidism.
- Pseudohypoparathyroidism.
- Hyperthyroidism.
- Growth hormone excess.
- Increased body fluid overload.
- Hyperalimentation.
- High phosphorus laxative.
- High phosphorus enema.
- Blood transfusion.
- Massive cell necrosis or destruction.
- Hypoxia.
- Hyperthermia.
- Crushing injuries.
- Cytotoxic therapy.
- The dangerous value is < 1.0 mg/dL.
What is the differential diagnosis of Phosphate and Calcium in various diseases?
| Diseases | Serum Phosphorus | Serum Calcium | Serum Alkaline phosphatase |
| Ectopic PTH syndrome | Low | High | High |
| Renal acidosis | Normal to low | Low to normal | High |
| Osteomalacia | Low to normal | Low to normal | High |
| Paget’s disease | Normal | Normal | High |
| Metastatic cancer to bone | Normal | Normal to high | Normal to high |
| Osteoporosis | Normal | Normal | Normal |
|
|
|
|
| Sprue | Normal to low | Low to normal | High |
| Hyperthyroidism | Normal to high | Normal to high | Normal to high |
| Sarcoidosis | Normal | Normal to high | High |
| Vitamin D excess | Normal to low | High | Normal to high |
Questions and answers:
Question 1: When phosphorus is low in the body?
Question 2: What is the role of antacids on the absorption of phosphorus.