Iron Total (Fe), Total Iron
Iron Total (Fe)
What sample is needed for Iron Total (Fe)?
- The test is done on the patient’s serum.
- Collect the blood sample in the morning.
- Avoid food at least 12 hours before giving the blood.
What are the Indications for Iron Total (Fe)?
- This test is done to evaluate the concentration of iron in the body.
- This test will give information about the deficiency or overdose of iron.
- It is advised during anemia workup.
What are the precautions for Iron Total (Fe)?
- Avoid hemolysis because iron of the RBCs may increase the iron level.
- Please get the history of blood transfusion in a recent period of time.
- The hemolytic disease may give a falsely high value.
- The Recent History of iron-containing food or medication will affect the result.
- Get the history of drugs that may decrease the value, like chloramphenicol, methicillin, colchicine, ACTH, testosterone, and deferoxamine.
- Get the history of drugs that may increase the level of iron, like Estrogen, dextran, ethanol, iron preparation, methyldopa, and oral contraceptives.
What are the most common terms used for Iron?
- Ferritin is the chief iron-storage protein in the body.
- Iron serum reflects iron level Fe+++ bound to transferrin, not free Hb in serum.
- Transferrin transports the Fe+++ molecules. Normally, 1/3 of iron-binding sites are occupied; the rest is called unsaturated iron-binding capacity.
- Iron-binding capacity (TIBC) is obtained by this formula = transferrin (mg/dL) x 0.025.
- Heme is a Greek word that means Haima (blood). It is a component of hemoglobin and a red organic pigment containing iron with which Oxygen can be combined. Its main function is to transport the oxygen.
Pathophysiology of Iron Total (Fe)
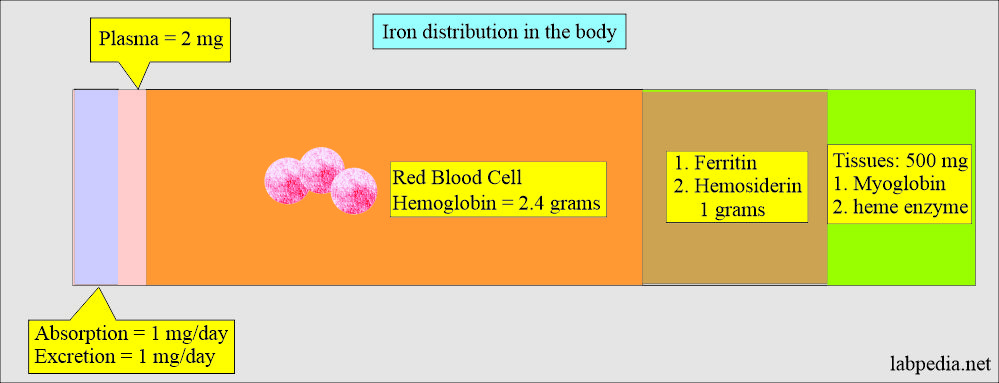
What is Iron distribution in the body?
- Iron is an essential component of heme protein that functions in oxygen transport or as an enzyme in the oxidation-reduction process.
- Iron is also a component of cytochrome, an enzyme that acts as an electron transfer agent in the oxidation-reduction process.
- Iron is a trace element present in the body. Normally, there is a very small amount of iron in the cells, plasma, and other body fluids.
- Iron is distributed in the body in different compartments like:
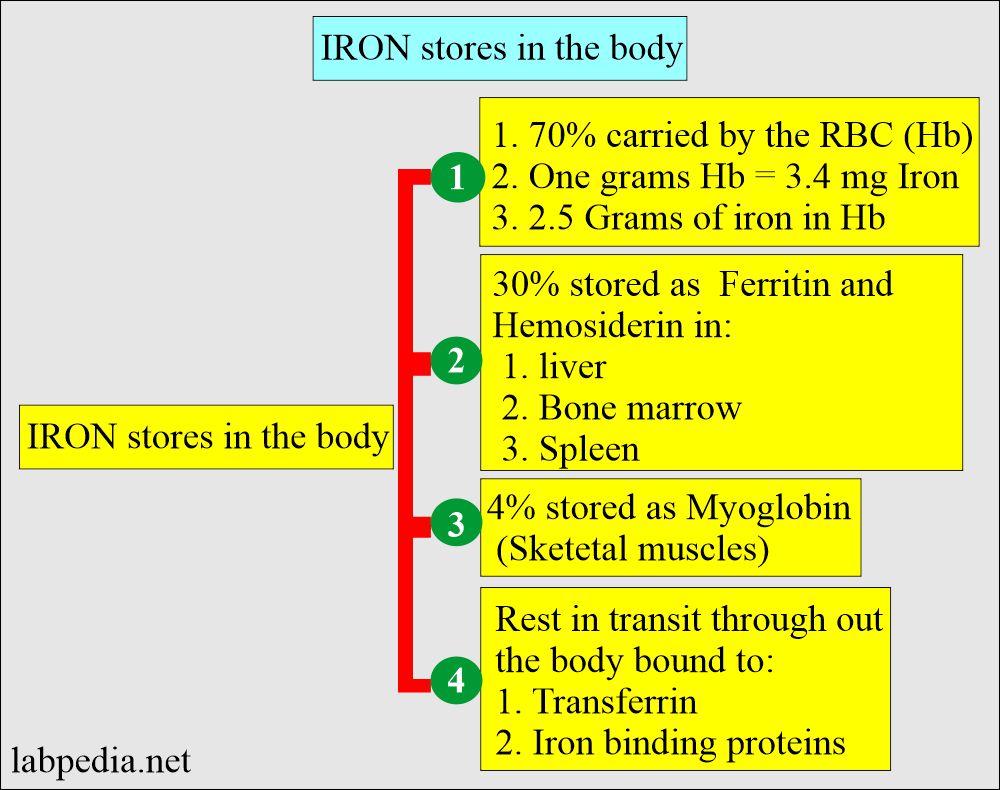
- Hemoglobin (70% of the body).
- One gram of hemoglobin = 3.4 mg of iron.
- One mL of packed RBCs contains 1 mg of iron.
- Approximately 2.5 G of iron is present in hemoglobin.
- Tissue iron.
- Myoglobin.
- Labile pool.
- The other 30% is present in the form of ferritin and hemosiderin.
What are the various facts about Iron distribution?
| Iron | Iron facts |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
How will you discuss the metabolism of iron (Fe)?
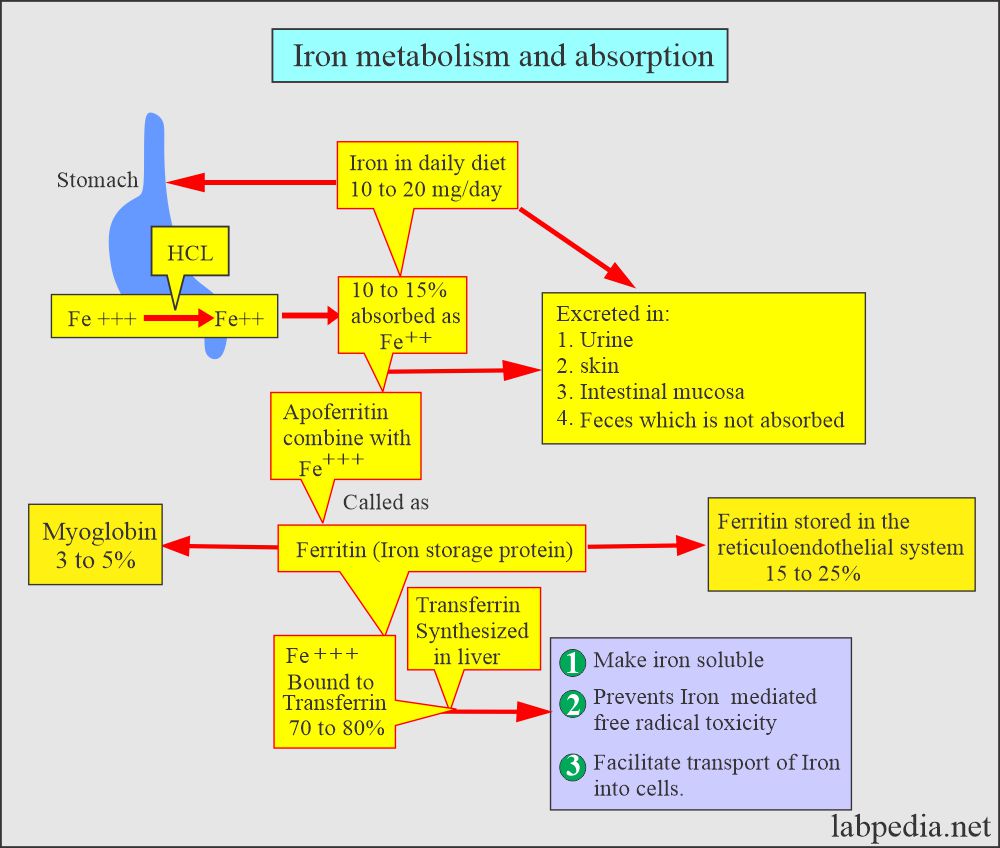
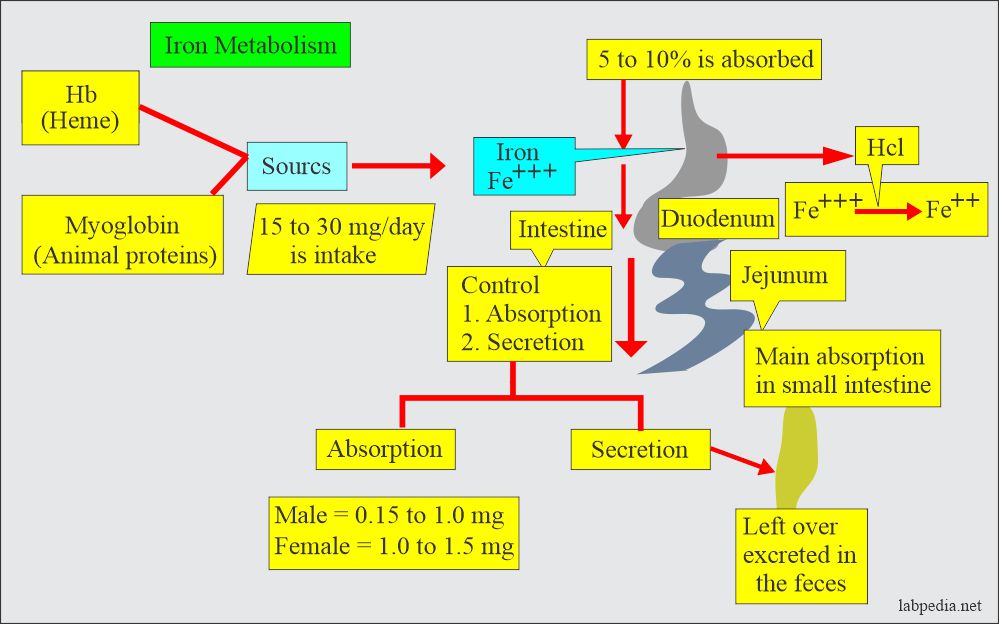
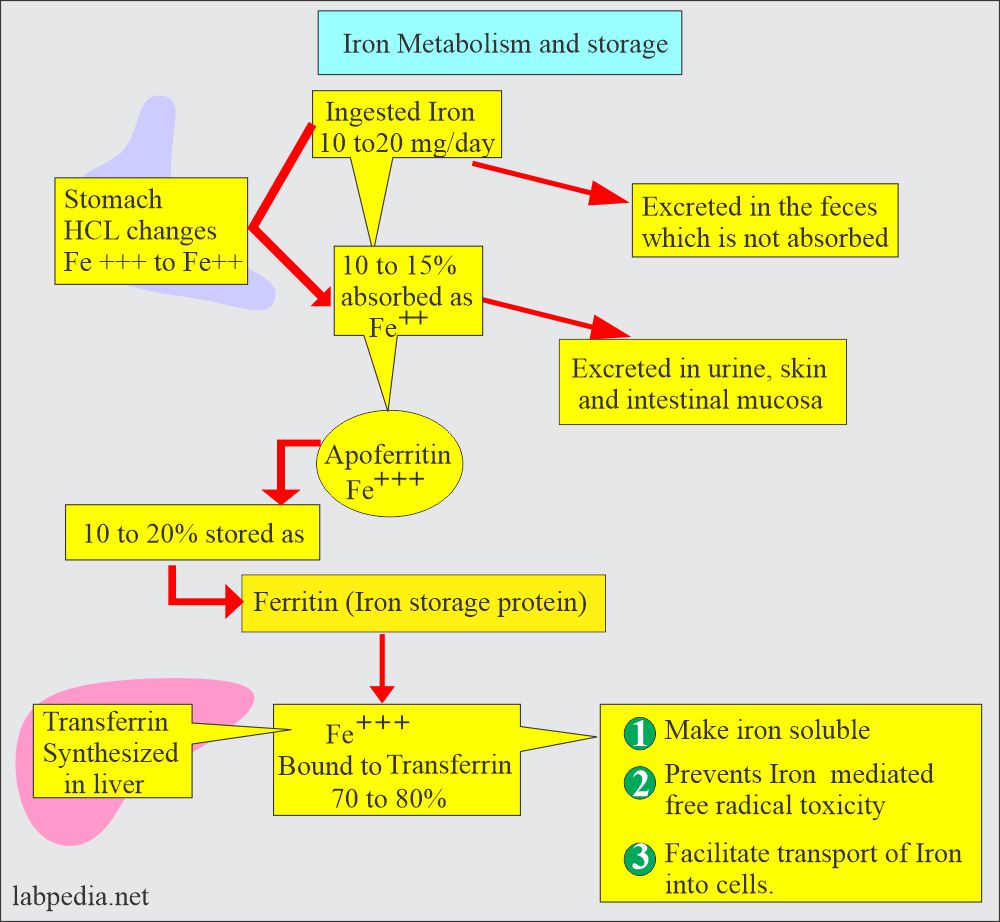
- Iron intake averages 10 mg/day, and around 10% is absorbed.
- Compared with food, around 50% or more of oral iron is absorbed if taken 45 to 60 minutes before food intake.
- Meat inhibits iron absorption less than dairy products or food with high fiber content.
- Coffee and tea inhibit iron absorption.
- Vitamin C increases iron absorption.
- Iron in human milk is more easily absorbed than the cow’s milk.
- Average iron loss in men is 1 mg/day and 2 mg/day in menstruating women.
- There is an additional requirement for pregnant and lactating mothers.
- In plasma, a total amount of 2.5 mg of iron is present.
- Iron is taken in the ferric form (Fe+++) in the diet and changed to the ferrous form (Fe++) in the stomach by Hydrochloric acid.
- It is then absorbed mainly in the small intestine (Duodenum and jejunum).
- The leftover is excreted in the feces.
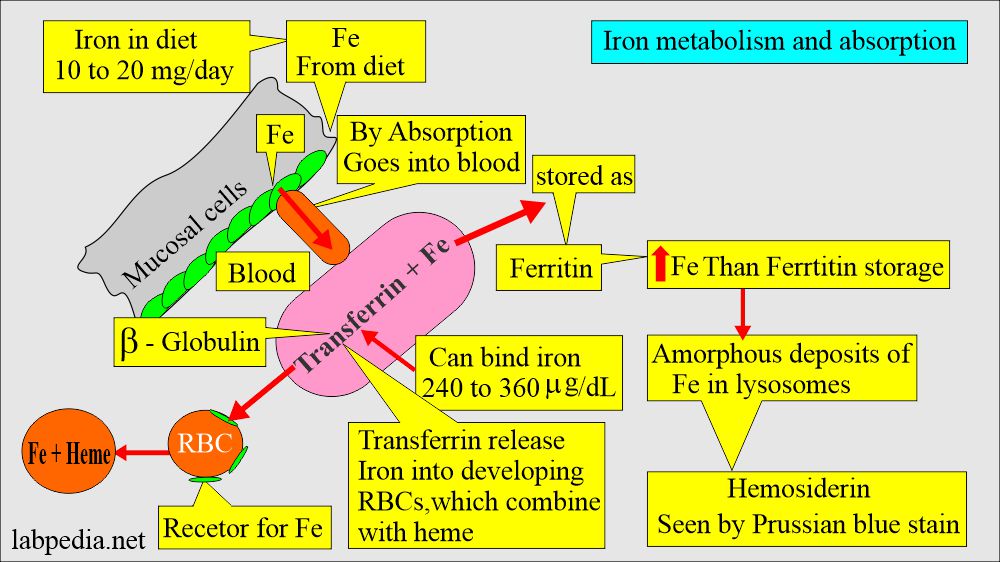
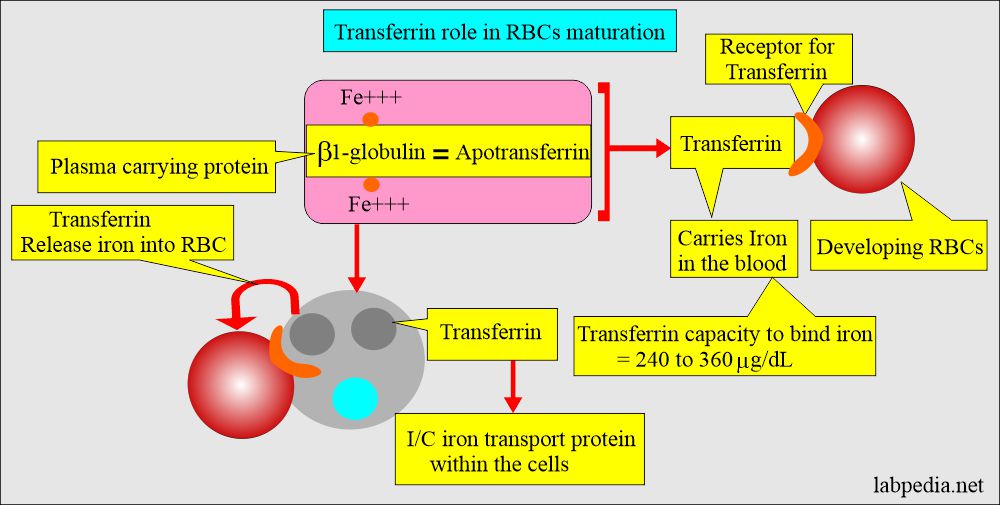
- Iron combines with the β-globulin and forms Transferrin, which circulates in the blood circulation.
- Iron, available in the blood, is used by the RBCs in the bone marrow.
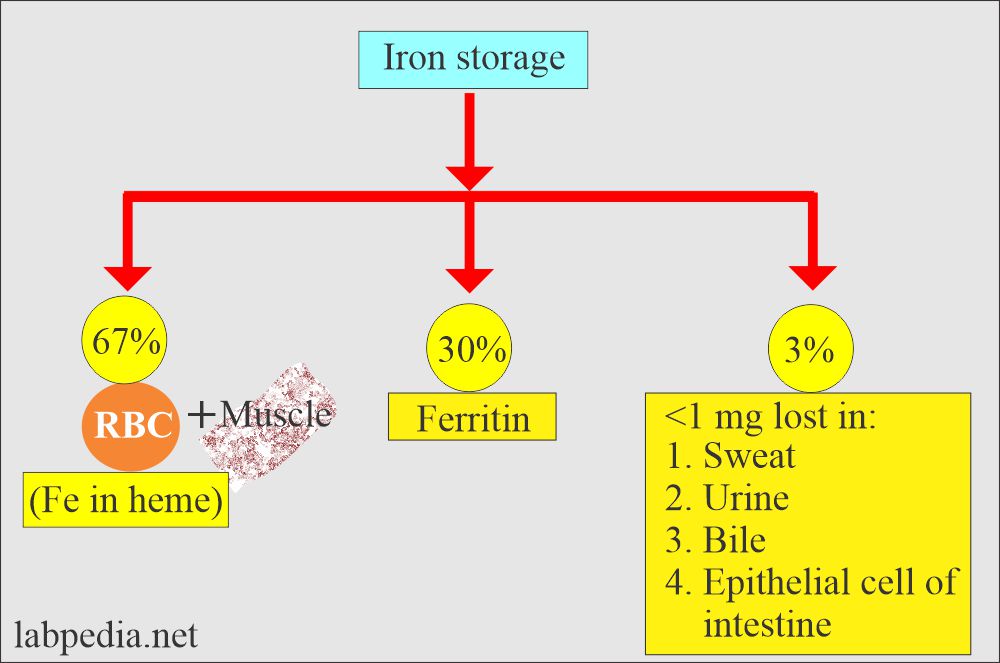
- 60% is stored as ferritin in the reticulum cells of bone marrow, liver, and spleen, and 40% is stored as hemosiderin.
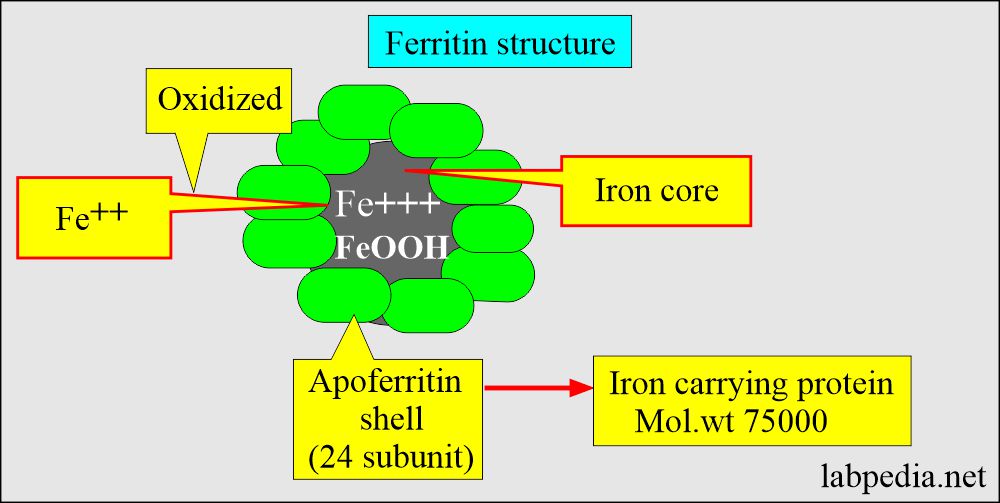
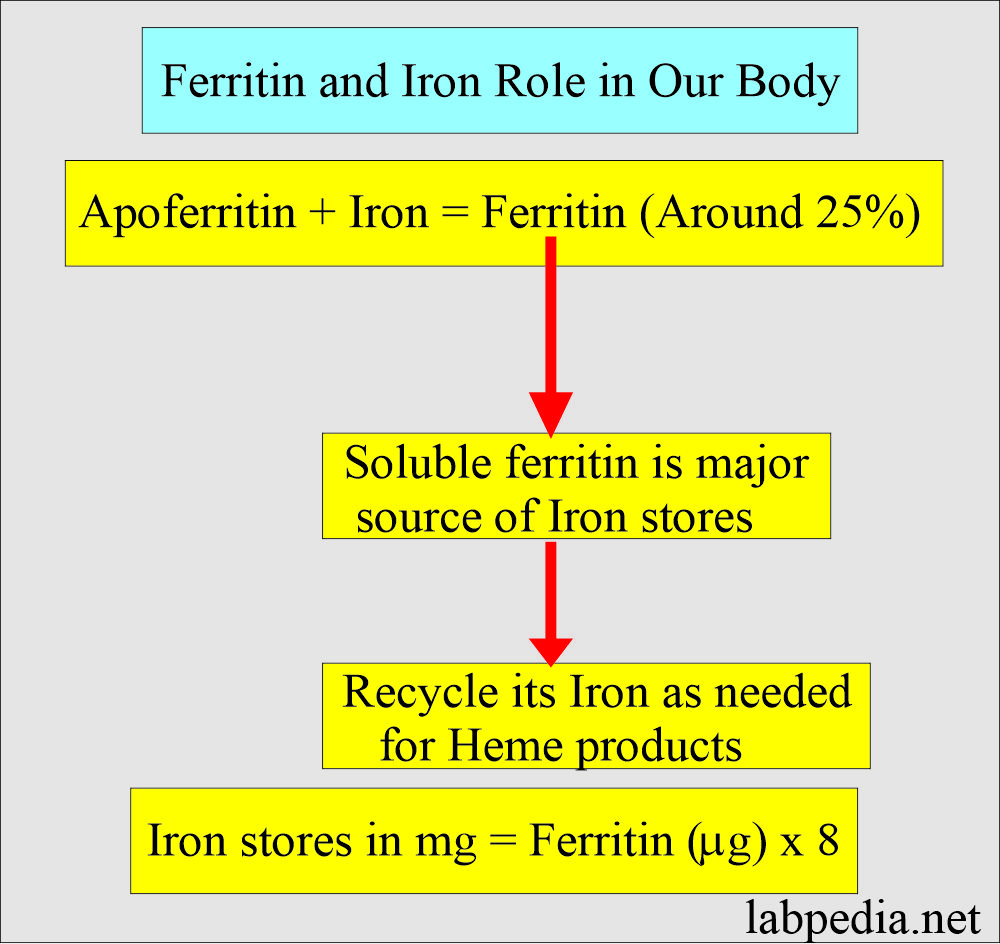
- It then combines with apoferritin, the protein that makes a ferritin complex.
- Iron is stored as ferritin in the body.
- Ferric ions combine with the Transferrin, which is synthesized in the liver.
- Transferrin helps:
- Make an insoluble iron form.
- It prevents iron-mediated free radical toxicity.
- This facilitates iron transport into the cells.
How is iron transported?
- Plasma protein apo-transferrin transports iron from one organ to another organ.
- This apo-transferrin is beta 1-globulin. It has two sites to attach to the iron.
- Apoferritin + Fe complex is called Transferrin (Iron + beta-glubolin = Transferrin).
Ferritin:
What is the main function of ferritin?
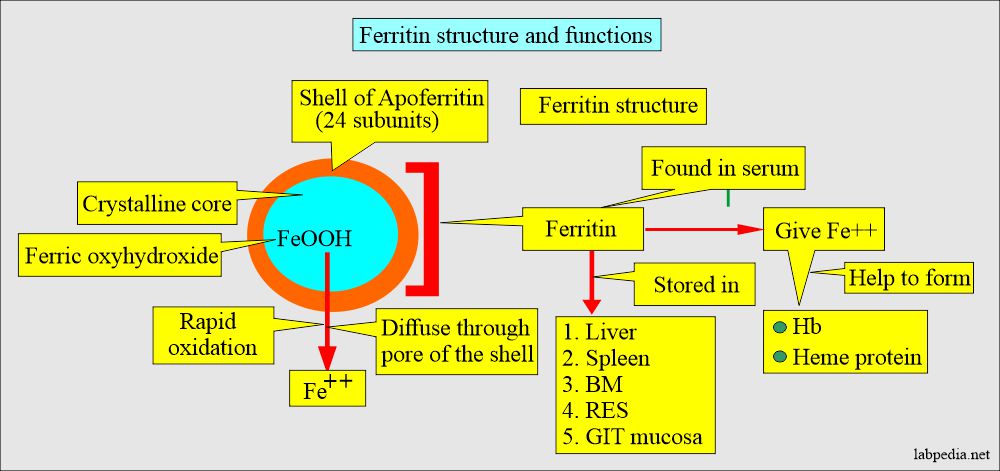
- It is the storage form of iron = Apoferritin shell + ferric oxyhydroxide FeO(OH).
- Ferritin is found in almost all cells of the body.
- Iron is supplied in the diet, and 10% of ingested iron is absorbed in the small intestine and transported to plasma.
- Iron in plasma is bound to β-globulin called Transferrin. It enters the bone marrow and is incorporated into the development of red blood cells, which become part of the hemoglobin.
- Ferritin in liver cells and macrophages is the reserve for hemoglobin and another hemoprotein.
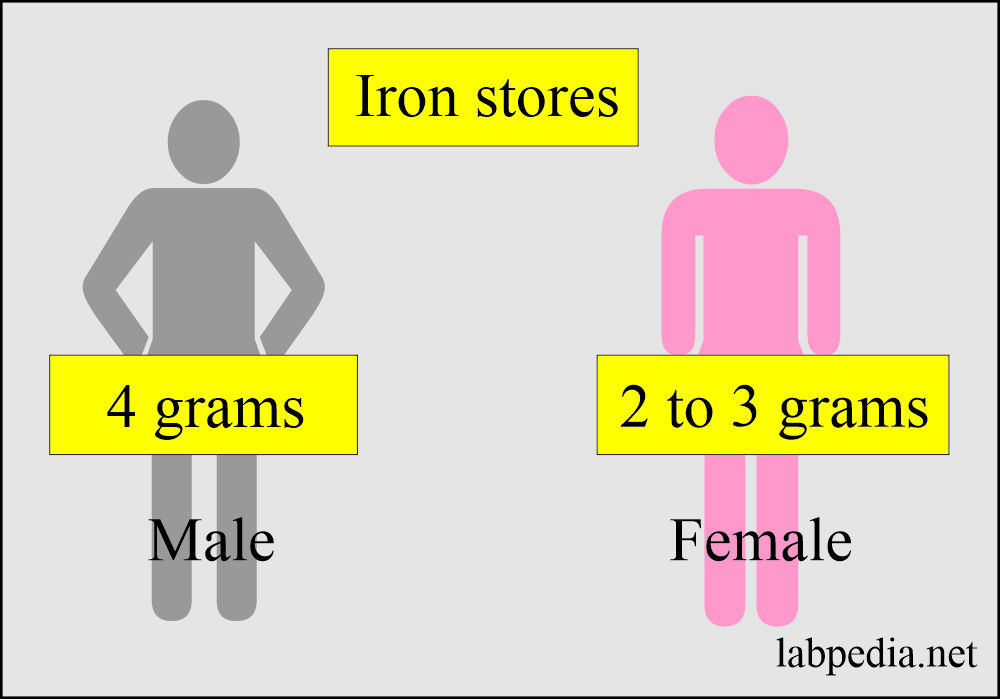
- Men’s total ferritin store is 800 mg.
- Women’s total ferritin stored varies from 0 to 200 mg.
- Ferritin concentration decreases before a drop in the hemoglobin and changes in the RBCs morphology or serum iron concentration.
Hemosiderin:
What is the main function of the Hemosiderin?
- It is also stored in the form of iron.
- This is aggregated, partially deproteinized ferritin.
- This is insoluble in the aqueous solution.
- This is found in the liver cells, spleen, and bone marrow.
- On-demand, it is released slowly.
- Iron is needed for the formation of hemoglobin.
What are the complications of iron overload/deficiency?
- Iron deficiency causes iron deficiency anemia.
- Overdose causes hemochromatosis.
- Iron overload is seen in:
- Hemosiderosis.
- Hemochromatosis is seen as an injury to the organs, and there is degeneration and fibrosis.
- Sideroblastic anemia is due to iron overload; no exact mechanism is known.
- 70% of iron is found in the hemoglobin of RBCs.
- 30% of iron is stored in the form of :
- Ferritin.
- Hemosiderin.
- 30% of iron is stored in the form of :
- Iron is supplied to the body through the diet, where 10% is absorbed in the small intestine (duodenum + jejunum) and delivered to the blood.
- Transferrin = Iron + globulin (Iron is bound to globulin).
- Transferrin goes to the Bone marrow and Forms hemoglobin.
- Serum iron is iron-bound to transferrin.
What is the normal Iron Total (Fe)?
Source 1
| Age | µg/dL | |
| Newborn | 100 to 250 | |
| Infant | 40 to 100 | |
| Child | 50 to 120 | |
| Intoxicated child | 280 to 2550 | |
| Fatally poison child | >1800 | |
| Adult | Male | Female |
| 65 to 175 | 50 to 170 | |
- To convert into SI unit x 0.179 = µmol/L
Source 2
- Male = 80 to 180 µg/dL.
- Female = 60 to 160 µg/dL.
- Newborn = 100 to 250 µg/dL.
- Child = 50 to 120 µg/dL.
What is the Normal urine iron?
- 100 to 300 ng/24 hours of urine
What is the Iron level in liver tissue?
- 530 to 900 µg/g dry weight of the liver tissue
What is the significance of lab tests of the total iron content?
- For iron deficiency, measurement of total iron, iron-binding capacity, and transferrin saturation should not be requested.
- The above tests are only useful in screening chronic iron overload diseases.
- Confirmation and monitoring of acute iron poisoning in children.
What are the conditions where there is increased Serum Iron Total (Fe)?
- Hemolytic anemias.
- Hemochromatosis or hemosiderosis.
- Multiple transfusions.
- An overdose of iron therapy.
- Nephritis.
- Liver damage and acute hepatitis.
- Vit.B6 deficiency.
- Lead poisoning.
- Acute leukemias.
- Iron overload syndrome.
What are the conditions where there is decreased serum Iron Total (Fe)?
- Iron deficiency anemia.
- Inadequate absorption of iron.
- Chronic blood loss.
- Paroxysmal nocturnal hematuria.
- Pregnancy is mostly in the third trimester.
- There is a 30% decrease in iron after every menstrual cycle.
- Chronic diseases, e.g., chronic infections, autoimmune diseases like SLE, and rheumatoid arthritis.
- Remission of pernicious anemia.
- Inadequate absorption from the intestine is like malabsorption.
- Short bowel syndrome.
- Malignancies.
- Chronic hematuria.
- Note: Serum iron should be advised along with total iron-binding capacity and transferrin.
Please see more details on Total iron-binding capacity and Transferrin.
Questions and answers:
Question 1: Which tests will not help in the diagnosis of iron deficiency anemia?
Question 2: What is the role of HCL in the absorption of iron?