Hepatitis C Virus (HCV)
What sample is needed for the Hepatitis C Virus (HCV)?
- The best sample is the patient’s serums.
- The serum is stable for 7 days at room temperature for anti-HCV.
- This test can be done on the plasma as well.
- This test can be performed on a random sample.
What are the precautions for the Hepatitis C Virus (HCV)?
- Separate serum or plasma immediately to avoid virus degradation by the white blood cells.
What are the indications for Hepatitis C Virus (HCV)?
- To diagnose the patient with HCV infection.
- For screening of the blood donor.
- This should be done to healthcare workers.
- Advised for the drug users.
- In a person who has sex with a positive partner.
- In patients with HIV positive.
- In abnormal liver function tests.
- Blood transfusion or organ transplantation before July 1992.
- The patients with hemophilia were treated before 1987.
How will you define the Hepatitis C Virus (HCV)?
- This was formally called non-A, non-B viral infection (NANB) because no tests were available. This was suspected by the exclusion of HBV and HAV.
How will you discuss the history of viruses NANB?
- Later on, the Hepatitis D virus was discovered, and this virus was separated from NANB.
- NANB was also known as short-incubation and long-incubation viruses.
- Short-incubation virus was labeled as Hepatitis E virus.
- The long-incubation name was still known as NANB.
- In 1991, the virus identified was named Hepatitis C Virus (HCV).
- The second-generation test for HCV was available in 1993.
- A third-generation test for HCV was available in 1994.
- First and second-generation tests diagnose only HCV-IgG.
- HCV is the only virus that was long-incubation and was known as NANB.
How will you discuss the biology and structure of the Hepatitis C Virus (HCV)?
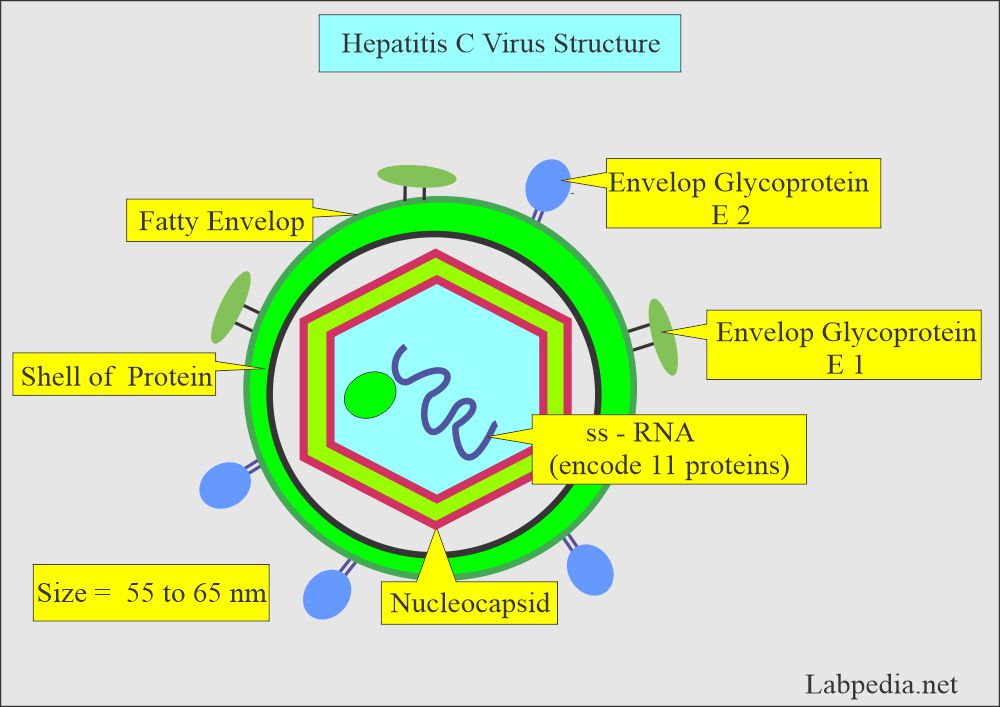
- Hepatitis C virus (HCV) is a hepatotropic virus. It belongs to the Flavivirus family.
- HCV is a small enveloped virus measuring 55 to 65 nm.
- HCV is a small, positive-single-stranded RNA virus of the Flaviviridae family.
- HCV consists of 10,000 nucleotides of single-stranded RNA molecules in one common open reading frame.
- This was formally called non-A, non-B viral infection because no test was available. This was suspected by the exclusion of HBV and HAV.
- HCV exists in 4 genotypes or strains:
- Core antigen.
- NS3 gene.
- NS4 antigen.
- NS5 antigen
- HCV infection is unlike HBV because this gives rise to more than 60% as a chronic disease.
- 20% of the patients develop cirrhosis and hepatocellular cancer.
What are the characteristic features of the Hepatitis C Virus (HCV)?
| Parameters | Characteristic features |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What is the genetic variation of the Hepatitis C Virus (HCV)?
- Genotypes of HCV are detected because of their response to treatment.
- There are 6 genotypes with several sub-types.
- Genotype 1 progresses to chronicity and cirrhosis. It is less responsive to treatment.
- Genotypes 2 and 3 are very responsive to antiviral treatment.
- 75% of Americans have genotype 1, subtypes 1a and 1b.
- 20% to 25% have genotypes 2 and 3.
- Genotype 4 is more common in African countries.
- Genotype 5 is more common in South Africa and Asia.
- Genotype 6 is more common in Southeast Asia.
- The smaller percentage has genotypes 4, 5, and 6.
What is the Hepatitis C Virus (HCV) genome structure?
- It consists of N- a terminal and a C-terminal.
- There are core proteins, envelope proteins, and non-structural proteins.
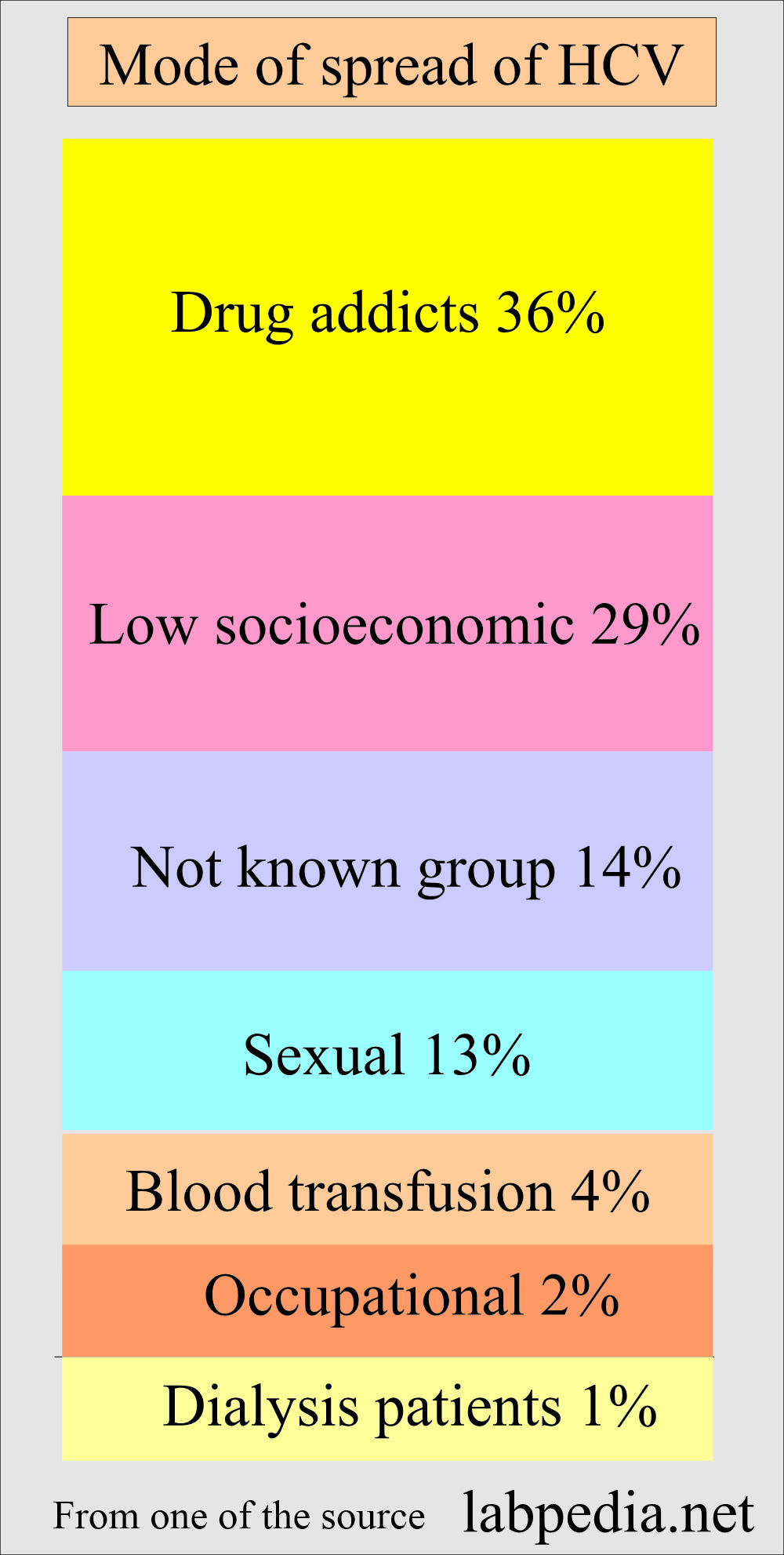
What is the mode of spread of the Hepatitis C Virus (HCV)?
- Blood (Serum).
- Saliva.
- Semen.
- Major risk groups are:
- Blood transfusion.
- I/V drug abusers.
- Blood products.
- The mode of spread is like HBV, with some differences.
- In 50%, the source of the cases is not known.
- One of the modes is blood transfusion or blood products (now rare because screening tests have been available since 1987).
- HCV hepatitis (post-transfusion hepatitis) is quite common in volunteer blood donors who are HBV-negative.
- Vertical transmission from mothers to newborns is not as common as HBV infection.
- This can spread through sexual contact, but some believe that sexual transmission is uncommon.
- This is seen in sex with multiple partners and unprotected.
- Male homosexuals are less likely to be infected as compared to HBV.
- It is also seen less in number in heterosexual groups.
- This is seen in organ transplantation (before 1992).
- This is common in I/V drug users.
- Tattooing in an unhygienic atmosphere.
- This may be seen in HIV patients.
- Sharing personal items like toothbrushes or shavers that may have blood contamination.
- It is believed that 50% of the case’s transmission mode is unknown.
- Fetal and neonatal transmission is uncommon.
- Passive transfer of anti-HCV antibodies is quite common.
What is the incubation period for the Hepatitis C Virus (HCV)?
- It is 2 to 12 weeks after exposure. (other references give 2 to 52 weeks) and the average period being 7 or 8 weeks).
- Incubation of 2 weeks to one year is also reported.
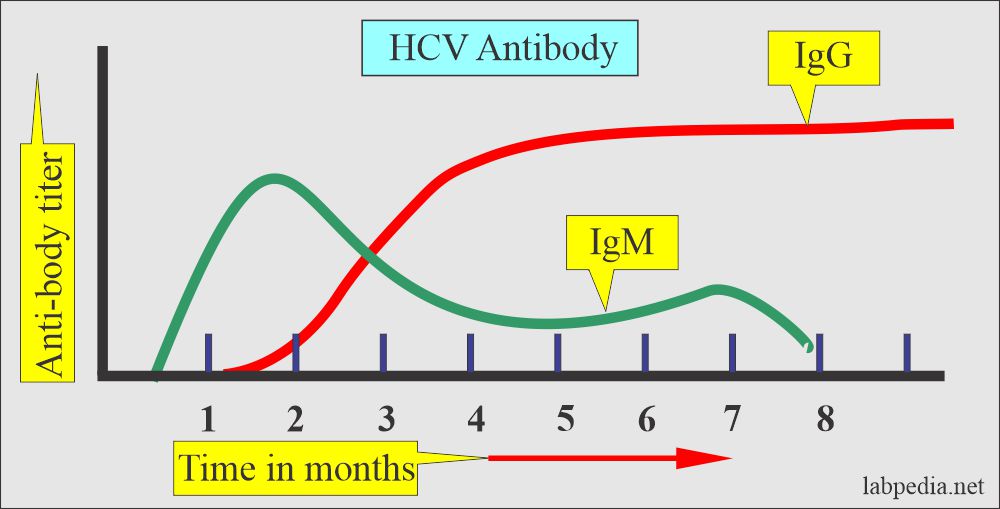
What are the types of antigens and antibodies of the Hepatitis C virus (HCV)?
- HCV-Ab (IgG) appears in 3 to 4 months. and disappears in 2 years.
- Most cases develop HCV-IgG antibodies by 6 weeks, and the range is 5 to 30 weeks after the onset of the symptoms.
- The anti-HCV can be detected in acute hepatitis C during the initial phase of elevated aminotransferase activity.
- This antibody may never be detected in 5% to 10% of the patients with acute Hepatitis, and anti-HCV may become undetectable after the recovery from acute hepatitis.
- The current screening test detects antibodies against HCV.
- An anti-HCV screening test has been available for less than 10 years.
- This is a unique virus in which, in acute infection, you can find an anti-HCV antibody and HCV-RNA antigen.
- Anti-HCV remains for many years. Therefore, a positive anti-HCV test indicates infection or carrier state but not infectivity or immunity.
What are the signs and Symptoms of the Hepatitis C Virus (HCV?
- HCV infection is also called a silent disease.
- The clinical S/S is like an HBV infection but less severe.
- It is in 12% to 25% of cases of sporadic hepatitis that is not related to parenteral inoculation or sexual transmission.
- Only 15% develop acute infection, and the rest go into a chronic disease.
- Chronicity of the infection is seen in 50% to 80% of the cases (another reference says 70 to 80%).
- Chronicity is more common than HBV infection.
- It is seen in 60% of post-transfusion cases and may take 10 years.
- 30% of cases may develop cirrhosis in around 10 years.
- HCV infection may lead to chronic active hepatitis and cirrhosis.
- In one of the studies, chronic active hepatitis was seen in <20% and 3% had cirrhosis.
- Fulminant hepatitis is seen in 1% to 2% of the patients.
- HCV infection symptoms may not appear or are very mild for years.
- Chronic infection is often asymptomatic.
- The silent disease still causes damage to the liver.
- The patient with the silent disease may come with chronic liver disease and may develop cirrhosis and liver cell carcinoma.
- It is considered a significant etiology for liver cell carcinoma.
What are the common symptoms of the Hepatitis C Virus (HCV)?
- Jaundice is seen in only 25% of acute hepatitis.
- Tiredness
- Loss of appetite
- Nausea and vomiting
- Abdominal pain
- Joints pain
- Fever
- dark urine and gray-colored stool
- Ultimately, the patient develops jaundice.
- This chronic virus infection can lead to liver cell carcinoma.
What is the Hepatitis C Virus (HCV) serological profile?
- An anti-HCV screening test has been available for less than 10 years.
- Anti-HCV Ab (IgG) indicates:
- Convalescent stage.
- Old HCV infection.
- Anti-HCV Ab (IgG) indicates:
- Anti-HCV antibodies are IgM and IgG types.
- Anti-HCV Ab seen against:
- Core antigen.
- NS3 gene.
- NS4 antigen.
- NS5 antigen.
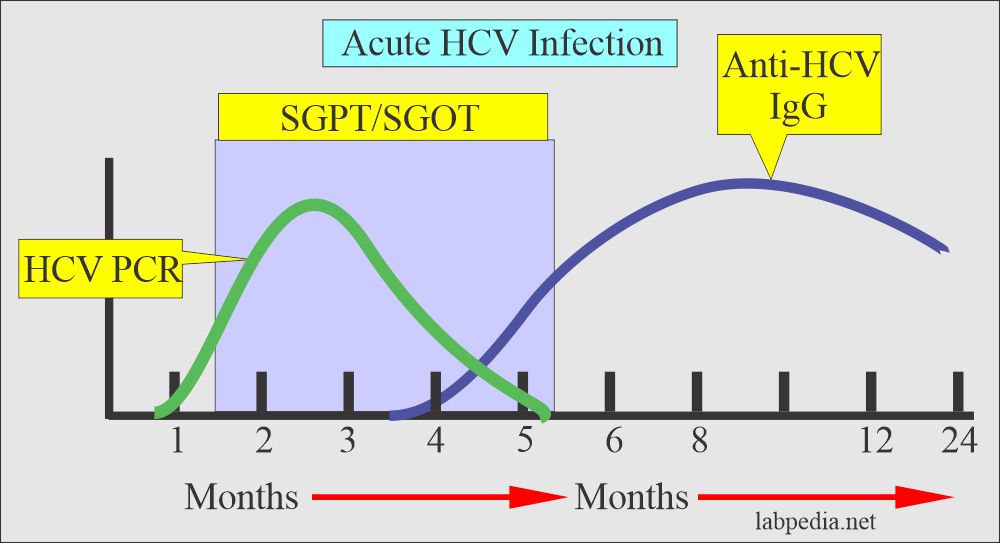
- HCV by PCR which detects HCV-RNA, is used to confirm the diagnosis.
-
- Viral load advises quantitative PCR.
-
- Usually, SGPT (ALT) is raised in this infection and chronic cases.
- A negative anti-HCV antibody does not exclude the HCV infection because seroconversion may not occur up to 6 months after exposure.
- A negative anti-HCV antibody does not exclude the HCV infection because seroconversion may not occur up to 6 months after exposure.
How will you diagnose the Hepatitis C Virus (HCV)?
- Interpretation of HCV profile:
- Acute infection = Anti-HCV antibody will be positive.
- Anti-HCV by ELIZA is confirmatory.
- PCR can confirm the diagnosis.
- Qualitative PCR for HCV genome.
- Quantitative HCV RNA PCR.
- PCR (polymerase chain reaction) will show the virus’s presence in the blood.
- PCR is the confirmatory test for HCV infection.
- PCR is done in patients to start the treatment.
| Clinical features of Acute HCV infection |
|
| Laboratory workup |
|
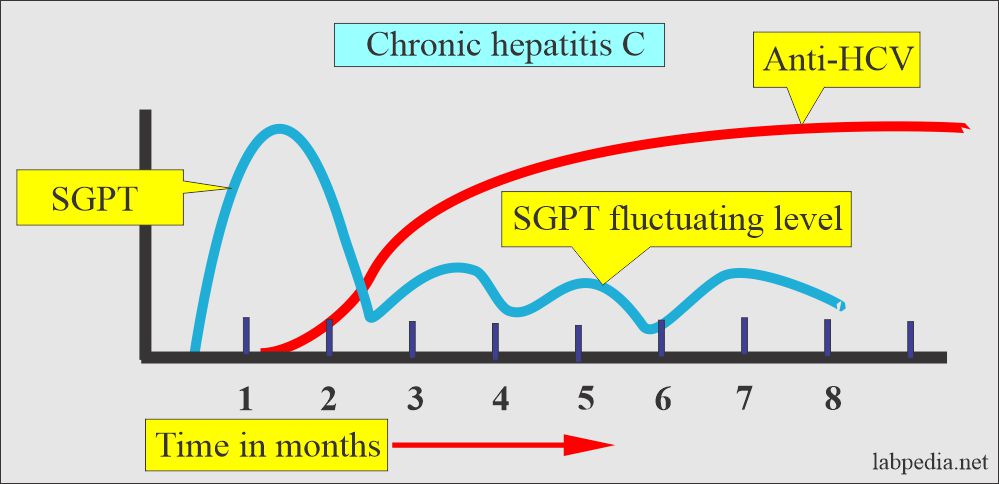
- Chronic infection = Almost 85 % shows anti-HCV antibody.
- Positive HCV-Ab indicates present or past infection.
| Clinical features of chronic HCV infection |
|
| Laboratory workup |
|
What are the HCV markers in various diseases?
| Test | Normal | Early infection | Acute | Chronic | Carrier | Recovery |
|---|---|---|---|---|---|---|
| Anti-HCV | Negative | Negative | Positive | Positive | Positive | Positive |
| PCR | Negative | Positive | Positive | Positive | Positive | Negative |
What are the complications of Hepatitis C Virus (HCV) infection?
- There are chances for:
- Fulminant hepatitis is seen in 1% to 2% of the patients.
- Cirrhosis is seen in 5% of the cases.
- Liver failure.
- Liver cancer risk is 15%.
- HCV infection is unlike HBV because this gives rise to more than 60% as a chronic disease.
- (some references say chronicity is from 50% to 80%)
How will you treat the Hepatitis C Virus (HCV)?
- Alfa-interferon alone. It gives benefits to <50% of the cases. Relapse is common when you stop the treatment.
- Interferon (IFN-α2, 3,000,000U) 3 doses per week is subcutaneously given for one year.
- It prevents the development of liver cell carcinoma.
- Monitor with SGPT, SGOT, and PCR for HCV.
- Retreatment can be given in 50 to 80% of the relapse cases.
- Type 2 and 3 genotype-positive patients are three times more sensitive to antiviral treatment like alpha-interferon or combination therapy, with the addition of Ribavirin.
- Alfa-interferon with Ribavirin as combination therapy.
- The latest therapy can cure 95% of patients.
- To label, that patient is cured when for three months PCR HCV is negative after the completion of the treatment.
How will you prevent the spread of the Hepatitis C Virus (HCV)?
- There is no vaccination available for HCV.
- Proper testing of the blood donors for transfusion for HCV has decreased the incidence.
Questions and answers:
Question 1: What is the chronicity in HCV infection as compared to HBV?
Question 2: Is there any possibility of vaccine for HCV?


Here you say anti hcv last 2 years then you write many years. What is true?
Thanks for the question. I have elaborated on the statement in the text. Please see the link.
https://www.labpedia.net/hepatitis-c-virus-part-1-hcv-profile/