Hemoglobin: – Part 1 – Hemoglobin (Hb) structure and Functions
Hemoglobin (Hb)
What sample is needed for Hemoglobin (Hb)?
- The blood sample is taken in EDTA.
- EDTA salt of sodium or potassium is the preferred sample.
- The blood sample is stable for 48 hours at 4°C and 24 hours at 23 °C.
What are the precautions for Hemoglobin (Hb)?
- Avoid clotting (micro-clots may form), which will lower the Hb.
- Falsely high results may occur due to prolonged venous stasis during venipuncture.
- Increased turbidity, paraproteins, and nucleated RBCs give high values.
- Lipemic blood also gives high values.
- There is a false low Hb in pregnancy due to increased blood volume.
- In the high altitude area, the Hb will be high.
- Gentamicin and methyldopa may increase Hb value.
- Antibiotics, chemotherapy, aspirin, and sulphonamide give low values.
What are the indications for Hemoglobin (Hb) estimation?
- It is done to diagnose anemia.
- It tells the severity of anemia.
- It will monitor the effectiveness of the treatment of anemia.
- This is done to evaluate polycythemia.
- This is a part of the complete blood examination.
How will you define Hemoglobin (Hb)?
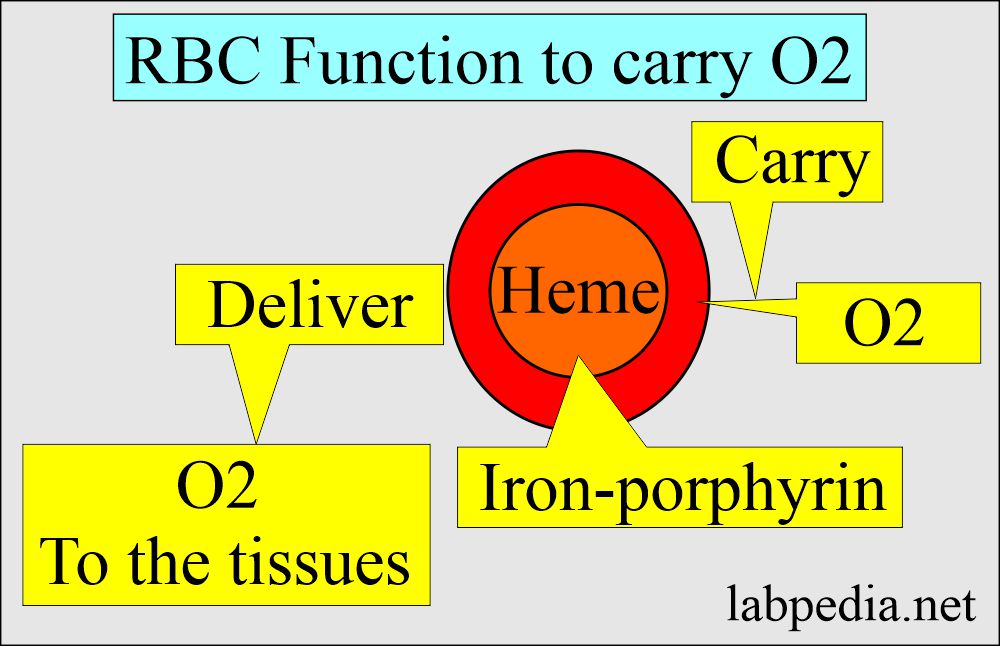
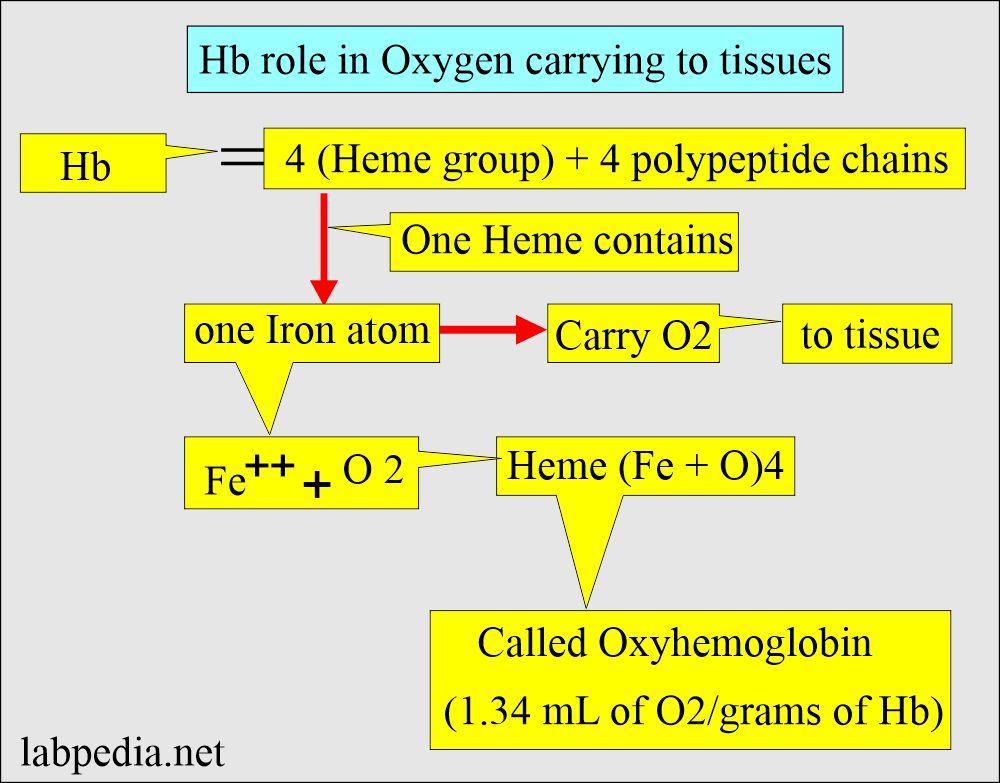
- Hemoglobin is an oxygen-carrying compound present in the RBCs.
- Total hemoglobin depends upon the number of RBCs in the blood.
- RBCs are the hemoglobin carriers.
- Hemoglobin is a hemoprotein whose primary function is transporting oxygen from the lungs to the body tissues.
- It was first isolated in 1849.
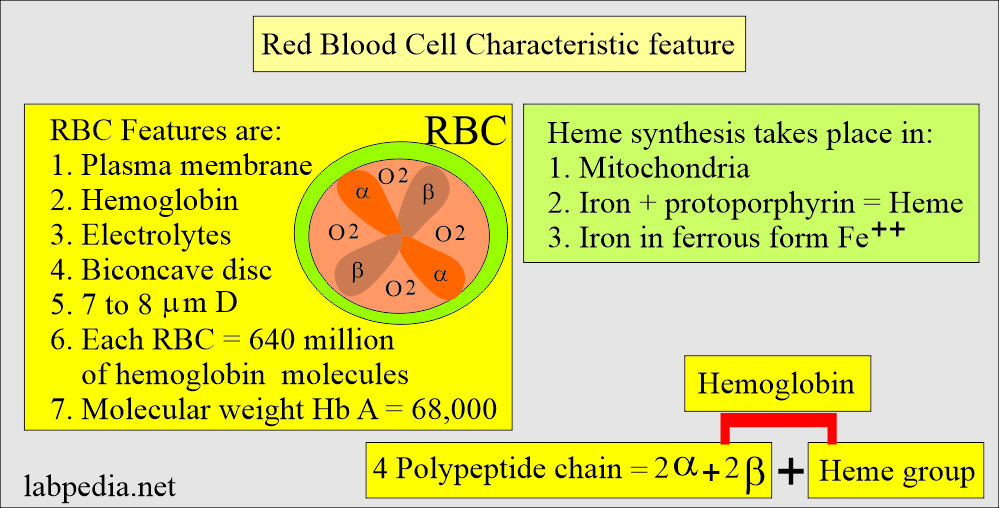
What is the structure of Hemoglobin (Hb)?
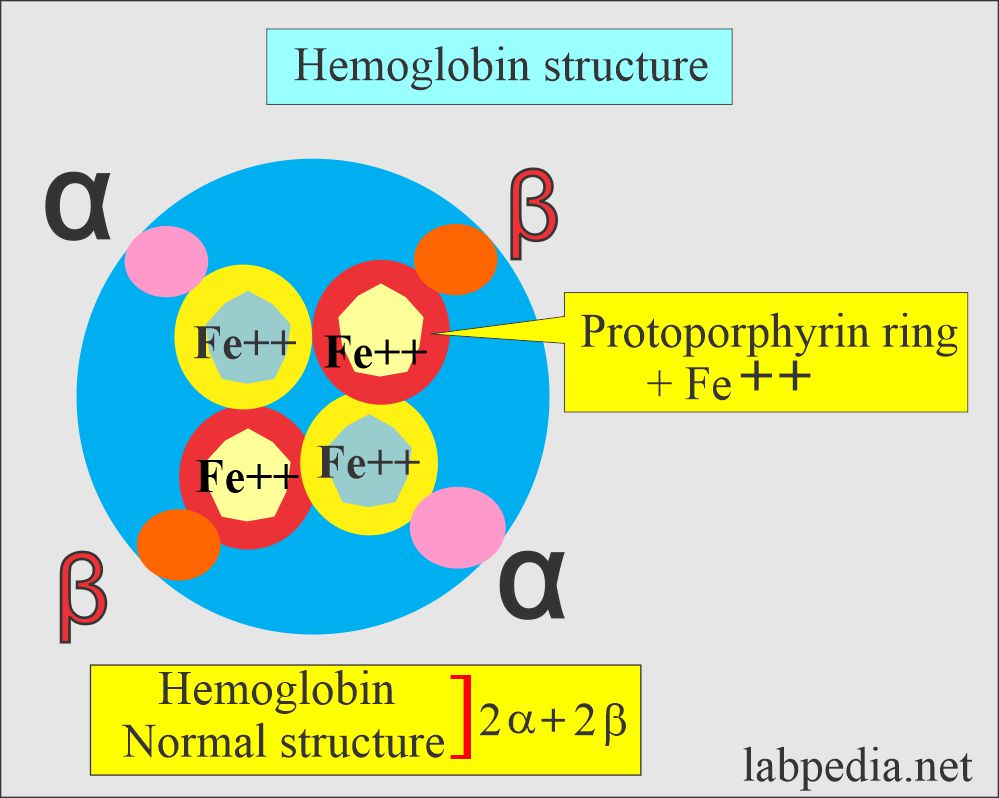
- Hb is a globular protein with a diameter of 6.4 nm in diameter.
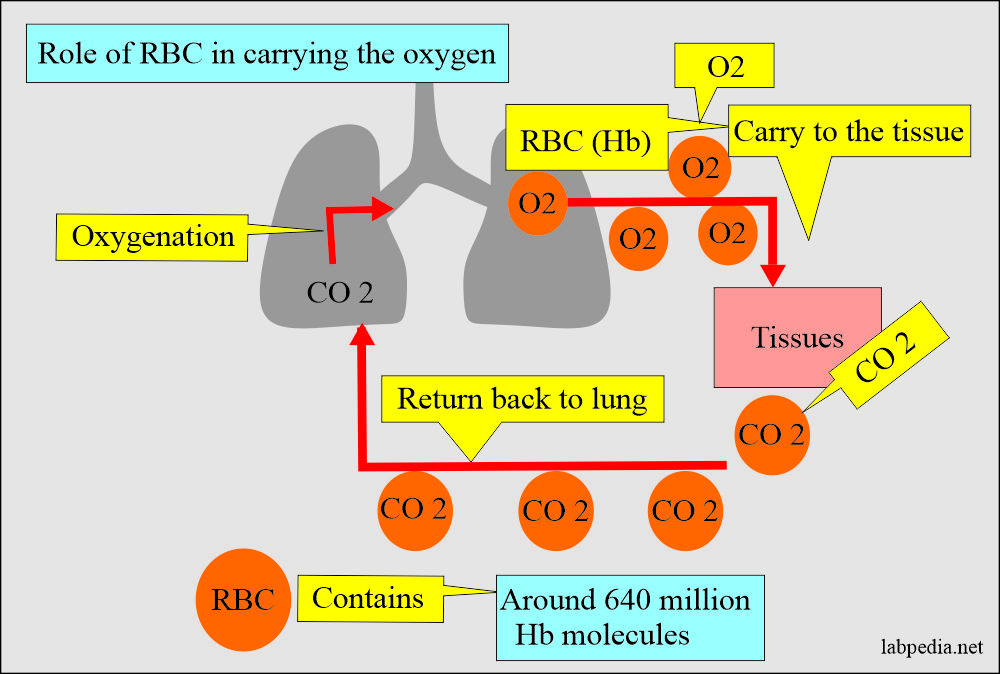
- Hb A has a molecular weight 68,000, and one RBC contains 640 million Hb molecules.
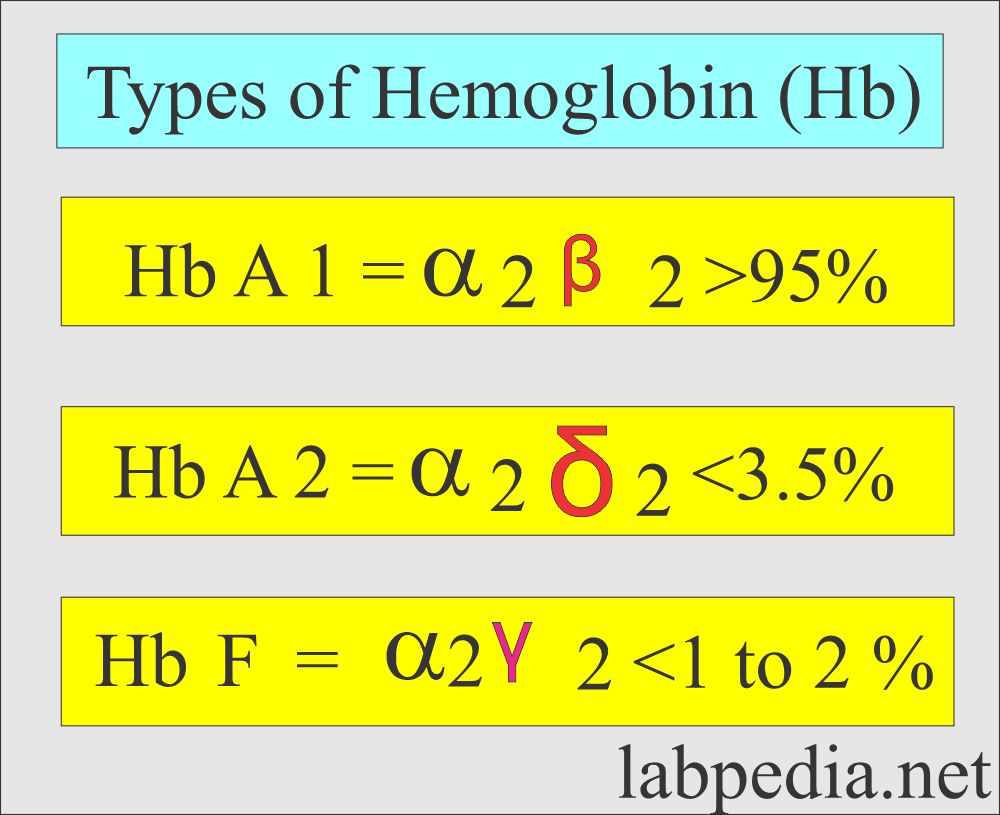
- Hb comprises four polypeptide chains, 2α and 2β, and heme groups. The 4 iron atoms are in the ferrous state (Fe++).
- Heme synthesis takes place in the mitochondria.
- The blood sample contains 96% of the Hb.
Hemoglobin normal structure
Red blood cell structure and characteristic feature:
| Age or stage of the fetus | Hb type | Structure |
|
|
|
|
|
|
|
|
|
- Zeta (ζ) chain production ceases at the gestational age of around 4 months.
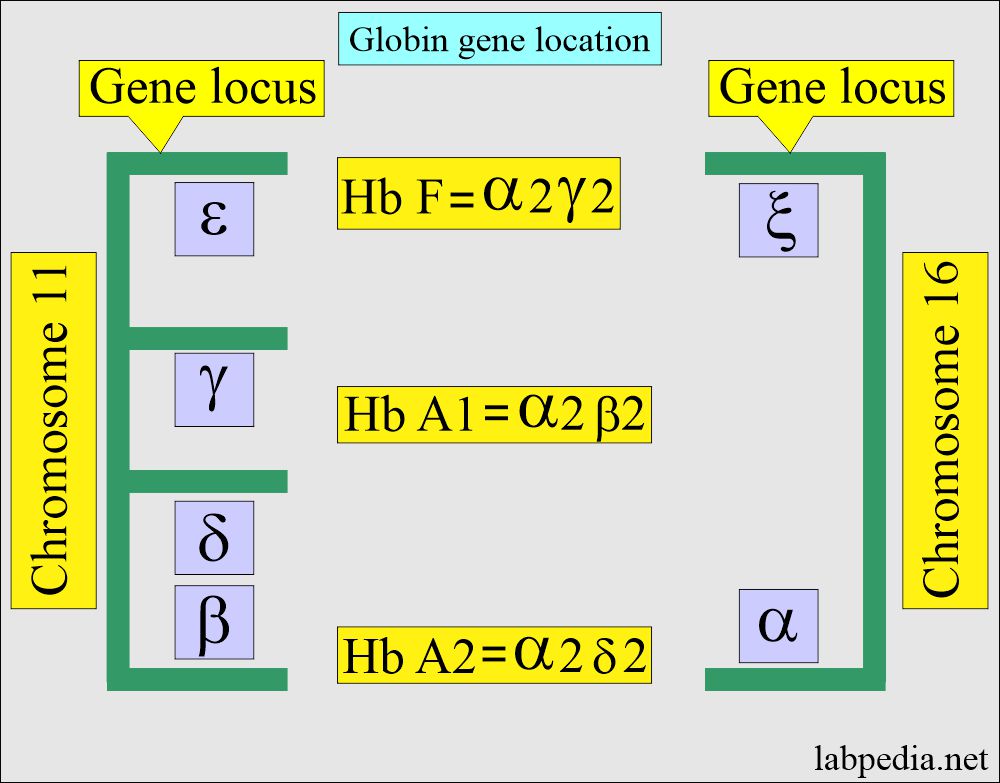
- Production of α-chain and β-chain starts at about the 6th week of gestation. Hb F (α2 γ2) increases in concentration, becoming the main hemoglobin in the fetus.
- Hb A (α2β2) starts at the gestational age of 28 weeks and can form up to 15% of the total hemoglobin at birth. The rest is mainly HbF and a very small amount of Hb A2.
- Production of the γ-chain decreases after birth, and normal adult, Hb F values, are obtained by one year of infants. In some cases, it may be raised for 2 years.
- The environment and chemicals modify a hemoglobin molecule, and the types are:
- Carboxyhemoglobin.
- Methemoglobin.
- Sulfhemoglobin.
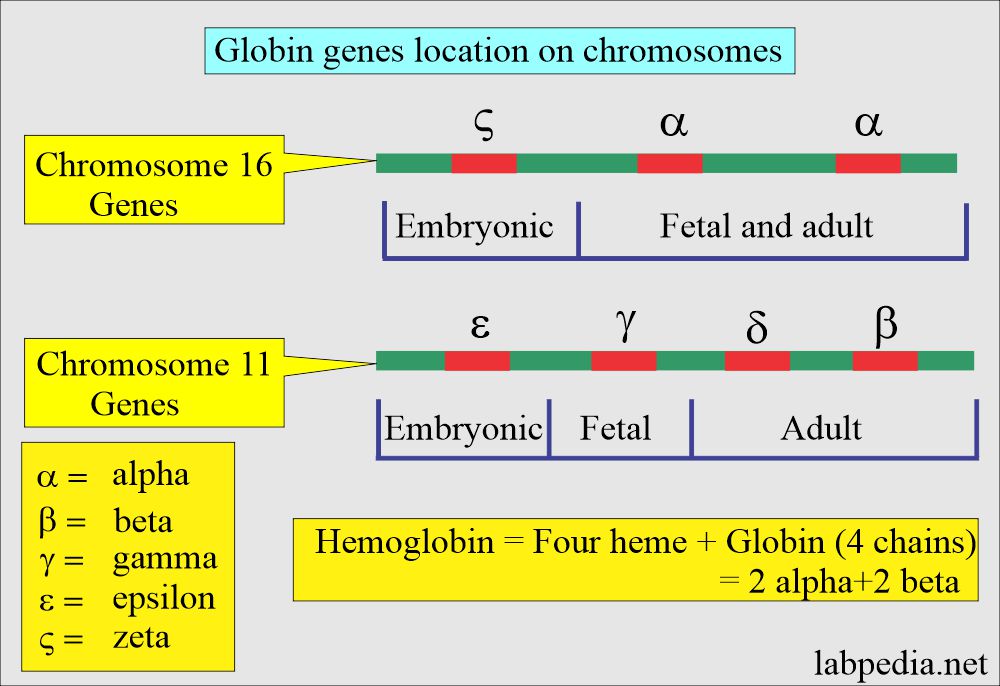
What is the location of the globin gene on the chromosome?
- The gene that controls α-like and ζ-globin chains is located in a cluster of chromosome 16 at position 16p 13.3 near the chromosome 16 telomere.
- The β, γ, and δ globins genes are clustered closely on chromosome 11.
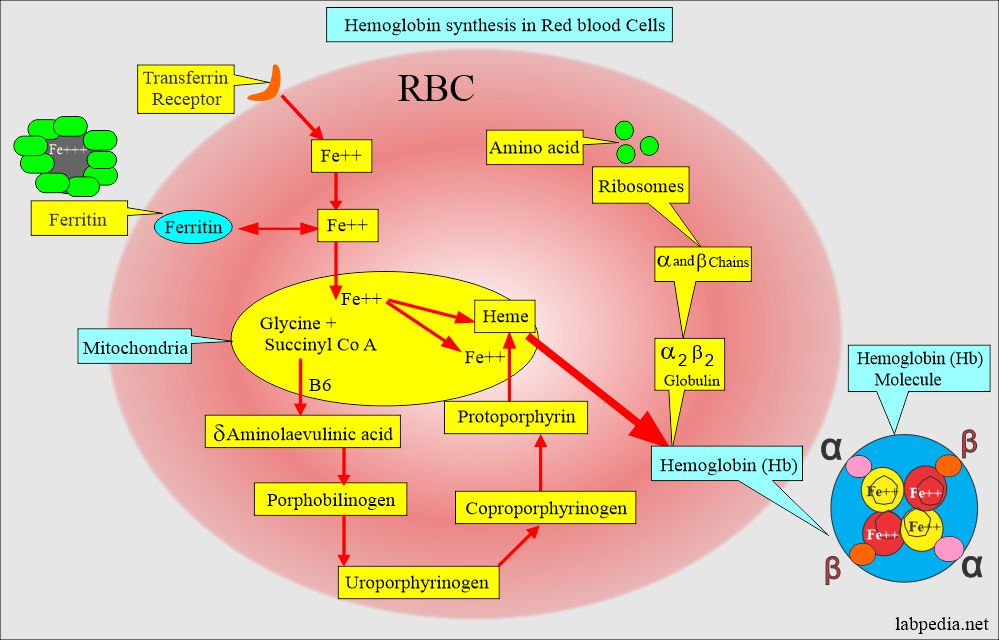
How will you discuss the synthesis of hemoglobin?
- The biosynthesis of hemoglobin requires the synthesis of heme and globin polypeptide chains.
- Hb consists of protein portions, such as Globin, and iron parts, such as heme.
- Hemoglobin synthesis is a complicated process. This takes place in the red blood cells.
- Amino acids are assembled in the ribosomes, giving rise to α2 β2 globulin.
- Transferrin gives Iron, which will combine with the haem molecule.
- The heme molecule will have protoporphyrin from the chemical combination of glycine and succinyl CoA in the presence of Vitamin B6 and form heme.
- Heme molecule consists of protoporphyrin IX protein and iron (Fe++).
- Protoporphyrin IX + Fe++ = Heme.
- Hb is the iron-containing pigment of the RBCs, carrying oxygen to various body parts.
- A central part of the Heme (iron-porphyrin) group is the site of Oxygen uptake and release.
What are the functions of Hemoglobin?
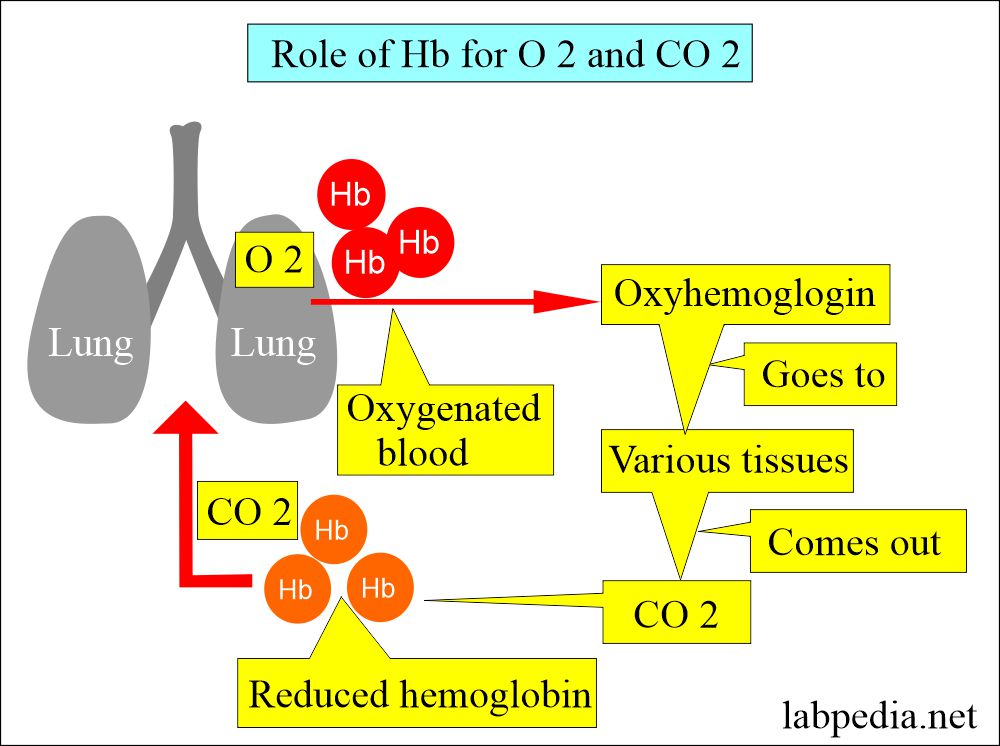
- Hb serves as the main vehicle for transporting oxygen and carbon dioxide.
- It carries oxygen from the lungs to tissues.
- CO2 from the tissue to the lung.
- It carries oxygen from the lungs to tissues.
- Iron combines with oxygen and gives it a red color.
- The oxygen combining capacity is directly related to Hb concentration, not the number of RBCs.
- The iron of heme is in Fe++ form, and it can combine irreversibly with oxygen and has a major role as an oxygen carrier.
- Hb acts as a buffer in the extracellular fluid (acid-base buffer system).
- In tissues, the oxygen concentration is lower, and CO2 and H+ ions concentration is higher.
- When the pH is lower, then oxygen dissociates from Hb.
- Now, deoxygenated Hb will bind to H ions and raise the pH.
- CO2 diffuses into RBCs and forms carbonic anhydrase, converting CO2 to HCO3¯ and protons.
- Protons are bound to Hb HCO3¯ ions and leave the cell.
- Every HCO3¯ ion leaving the cell will lead to the entry of a chloride ion.
- This buffer system depends on the lungs and kidneys to eliminate CO2 and HCO3¯.
- Abnormalities in the globin structure lead to hemoglobinopathies like Sickle cell anemia and Hb C disease.
- In thalassemia, the globin chain synthesis is abnormal.
- Hb closely reflects the hematocrit and RBC count.
- Hb at birth is usually lower in premature infants.
- There is no significant change in Hb concentration after 85 years.
- The main function of hemoglobin is transporting oxygen to the tissues and removing carbon dioxide.
What are the signs and symptoms due to Hb level?
- There are >800 variants of hemoglobin. Only 9 variants and thalassemia have significance.
- Low Hb:
- It puts a strain on the cardiopulmonary system to maintain the oxygen level.
- There is a risk of angina, heart attack, and congestive heart failure.
- Stroke.
- High Hb:
- The chances for intravascular settling.
- Stroke.
- Infarction in other organs.
What are the normal values of Hemoglobin?
Source 1
| Age | Hb g/dL | |
| Fetal Hb | ||
| 18 to 20 weeks | 11.7 ± 0.78 | |
| 21 to 22 weeks | 12.28 ± 0.89 | |
| 23 to 25 weeks | 12.40 ± 0.77 | |
| 26 to 30 weeks | 13.35 ± 1.17 | |
| Cord blood | 13.5 to 20.5 | |
| Infants | ||
| 0.5 month | 13.4 to 19.8 | |
| 1 month | 10.7 to 17.1 | |
| 2 month | 9.4 to 13.0 | |
| 4 month | 10.3 to 14.1 | |
| 6 month | 11.1 to 14.1 | |
| 9 month | 11.4 to 14.0 | |
| one year | 11.3 to 14.1 | |
| Child | ||
| 0.5 to 2 years | 11.0 to 14.0 | |
| 2 to 5 years | 11.0 to 14.0 | |
| 5 to 9 years | 11.5 to 14.5 | |
| 9 to 12 years | 12.0 to 15.0 | |
| Male | Female | |
| 12 to 14 years | 12.0 to 16.0 | 11.5 to 15.0 |
| 15 to 17 years | 11.7 to 16.6 | 11.7 to 15.3 |
| Adult | ||
| 18 to 44 years | 13.2 to 17.2 | 11.7 to15.5 |
| 45 to 64 years | 13.1 to 17.2 | 11.7 to 16.0 |
| 65 to 74 years | 12.6 to 17.4 | 11.7 to 16.1 |
- To convert into SI units x 10 = g/L
Another source
- Women = 12 to 16 g/dl
- Men = 14 to 17.4 g/dl
- Pregnant women = > 11 g/dl.
Another source
Normal Values in Infants and Children
| 0 to 2 weeks | 14 to 24.5 g/dl |
| 2 to 8 weeks | 12.5 to 20.5 g/dl |
| 2 to 6 months | 10.7 to 17.3 g/dl |
| 1 to 6 years | 9.5 to 14.1 g/dl |
| 6 to 16 years | 10.3 to 14.9 g/dl |
Normal value in Cord blood
- 9 month = 11.4 to 14 g/dl
- The normal values should be decided according to the population of various countries.
What are the Normal values of a different fraction of Hb in the adult?
- Hemoglobin A 2 = 1.5 to 3.5 % of the total Hb.
- Hemoglobin F = < 1% of total Hb.
- Hemoglobin in plasma = 0.5 to 5.0 mg/dL.
- Methemoglobin = < 1% of total Hb.
What are the critical values of Hemoglobin (Hb)?
- Blood transfusion is not recommended as long as the Hb is above 8 g/dl. and Hct is above 24 %.
- Hb < 5 g/dl is critical and needs a blood transfusion.
- Blood transfusion is recommended in older patients when the Hb level is below 10 g/dl.
- Critical value:
- When Hemoglobin <7 g/dL.
- When hemoglobin >20 g/dL.
What are the conditions where Increased Hemoglobin (Hb) is seen?
- Polycythemia.
- Polycythemia vera.
- Congestive heart failure.
- Chronic obstructive pulmonary disease (COPD).
- After vigorous exercise.
- Hemoconcentration, like dehydration, burns, and severe vomiting.
- Intestinal obstruction.
- Severe dehydration like diarrhea and burns.
What are the conditions where Decreased Hemoglobin (Hb) is seen?
- Anemia.
- Drugs that cause aplastic anemia.
- Drugs that cause hemolysis ( G6PD deficiency ).
- Immune hemolytic anemia.
- Iron deficiency anemia.
- Thalassemia.
- Pernicious anemia.
- Hemoglobinopathies.
- Liver diseases and Cirrhosis.
- Hypothyroidism.
- The hemorrhage is acute or chronic, like bleeding hemorrhoids or ulcers.
- Malignancies:
- Hodgkin’s disease.
- Leukemia.
- Lymphomas.
- Carcinomatosis.
- Multiple myelomas.
- Autoimmune diseases.
- SLE.
- Sarcoidosis.
- Rheumatoid arthritis.
- Dietary deficiency.
- Deficiency of iron.
- Deficiency of vitamin B12 and folic acid.
- Malabsorption syndrome.
Questions and answers:
Question 1: What is the main function of the hemoglobin?
Question 2: What is the role of hemoglobin in the extracellular space?