Chapter 5: Immunoglobulins and Their Properties
IMMUNOGLOBULIN CLASSES
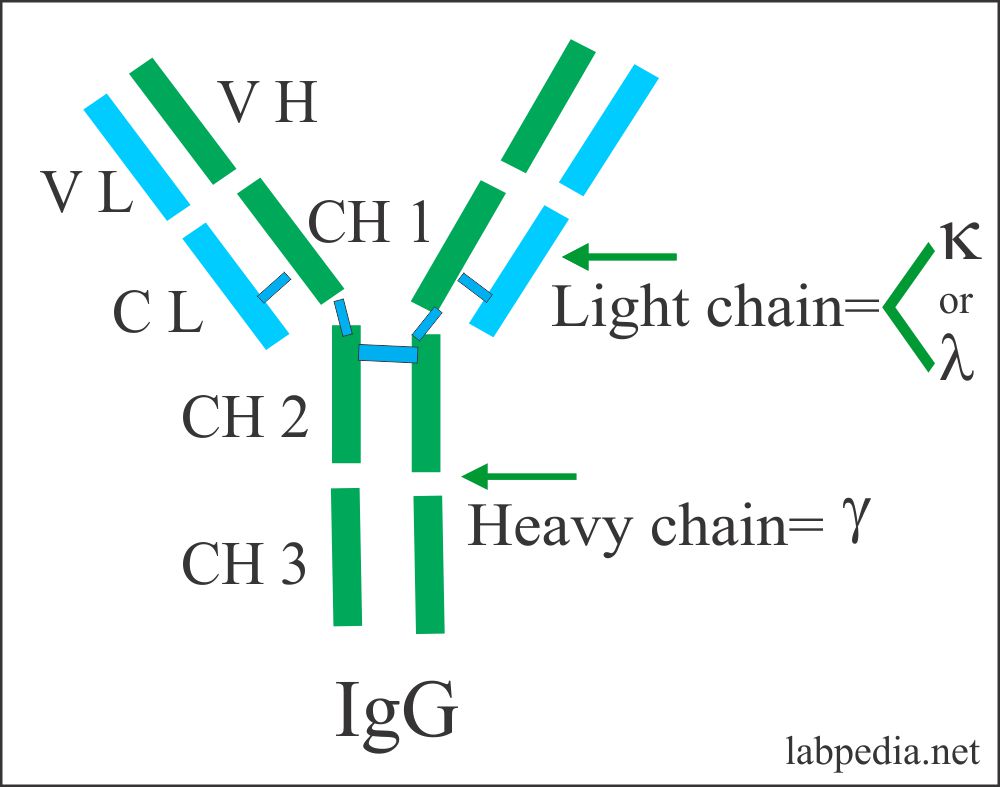
IgG
- IgG is the most abundant of all immunoglobulin, 75% of the total.
- 10G /L in adult (1200mg/dl)
- It consists of a monomer form.
- It has four subclasses
- IgG1
- IgG2
- IgG3
- IgG4
- It can activate complement.
- This is the major opsonin that helps in phagocytosis.
- The molecular weight is 150,000 to 170,000.
- It has a half-life of approximately 21 days.
- This is the only Ig that can cross the placenta in humans.
- It can cause the neutralization of toxins.
- It takes part in ADCC (Antibody-Dependent Cellular Cytotoxicity).
- It is 50/50 in extravascular and intravascular spaces.
- This is the immunoglobulin that diffuses readily into the extravascular spaces.
- IgG3 subclass is slightly larger than the other types with a molecular weight of 170, 000.
- Normal value = 800 to 1800 mg/dL (90 to 210 IU/mL).
- In infants 3 to 4 months of age = 350 to 400 mg/dL (40 to 45 IU/mL).
- By the end of the first year. It gradually increases to 700 to 800 mg/dL (80 to 90 IU/mL).
- Cord blood = 800 to 1800 mg/dL.
- CSF = 2 to 4 mg/dL.
- IgG increases in:
- Hepatitis, rubella, and infectious mononucleosis.
- Autoimmune diseases like rheumatoid arthritis, and SLE.
- Polyclonal gammopathy, monoclonal gammopathy, Hodgkin’s disease, and leukemia.
The subclasses of IgG have different functions and different serum concentrations shown in the following table:
| Features | IgG1 | IgG2 | IgG3 | IgG4 |
| 1. % of the total | 65 | 20 | 10 | 5 |
| 2. Activation of complement | ++ | + | ++ | – |
| 3. Placental transfer | ++ | ++ | ++ | ++ |
|
4. Binding for γ-receptor FcγR1 FcγR2 FcγR3 |
++ ++ ++ |
– – – |
++ ++ ++ |
+ – – |
|
5. Major antibacterial Response |
+ | ++ | + | + |
| 6. Anti- tetanus response | ++ | + | + | ++ |
IgG properties:
| Present in | Functions | Increased in | DEcreased in |
|
|
|
|
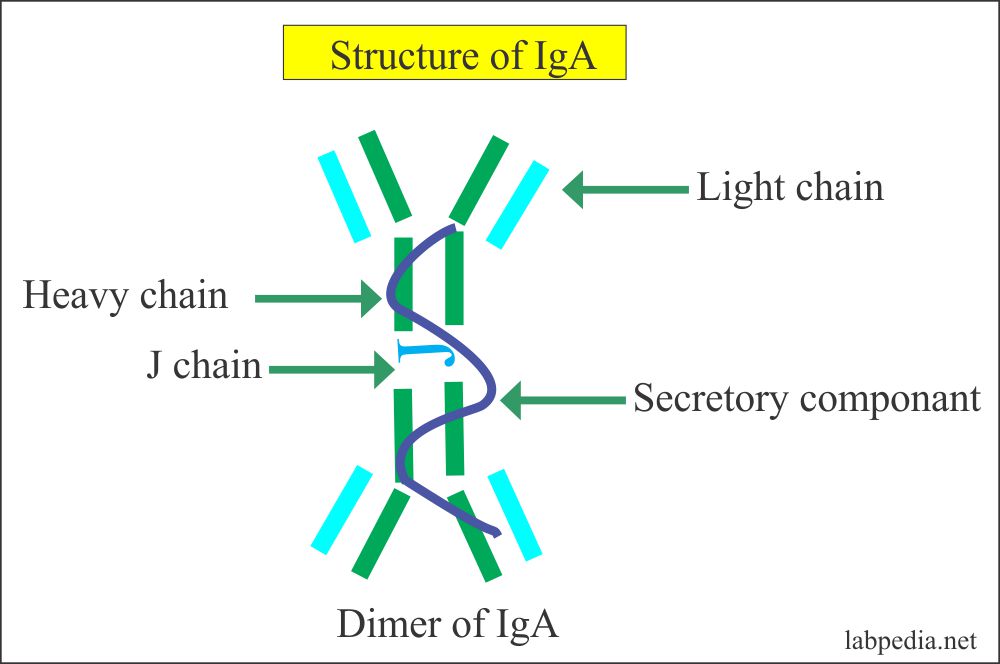
IgA
- IgA is the second most common Ig and two subclasses IgA1 & IgA2.
- It is 15% of the total Ig and the molecular weight is 400,000.
- It is present in abundance in saliva, tears, bronchial secretions, oral mucosa, prostatic fluid, vaginal secretions, small intestinal secretions, colostrum, and breast milk.
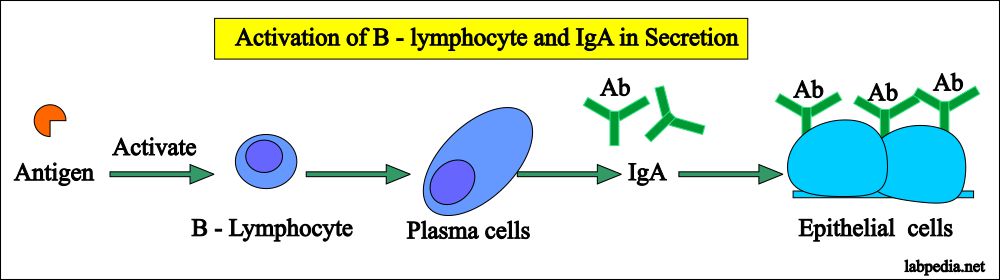
- It is synthesized by the plasma cells which are mostly present on the surfaces of the cells.
- It exists as a monomer and dimer form.
- In dimer from two monomers (IgA molecule) are joined by J-chain (short peptide).
- It has a half-life of 6 days.
- The Secretory IgA molecule consists of two 4-chain basic units. Joined by a short peptide the J- chain.
- It has an important role in the host defense of the warm and moist mucosal surfaces, which is achieved by secretory IgA.
- It is present in serum, usually in monomeric form (80%).
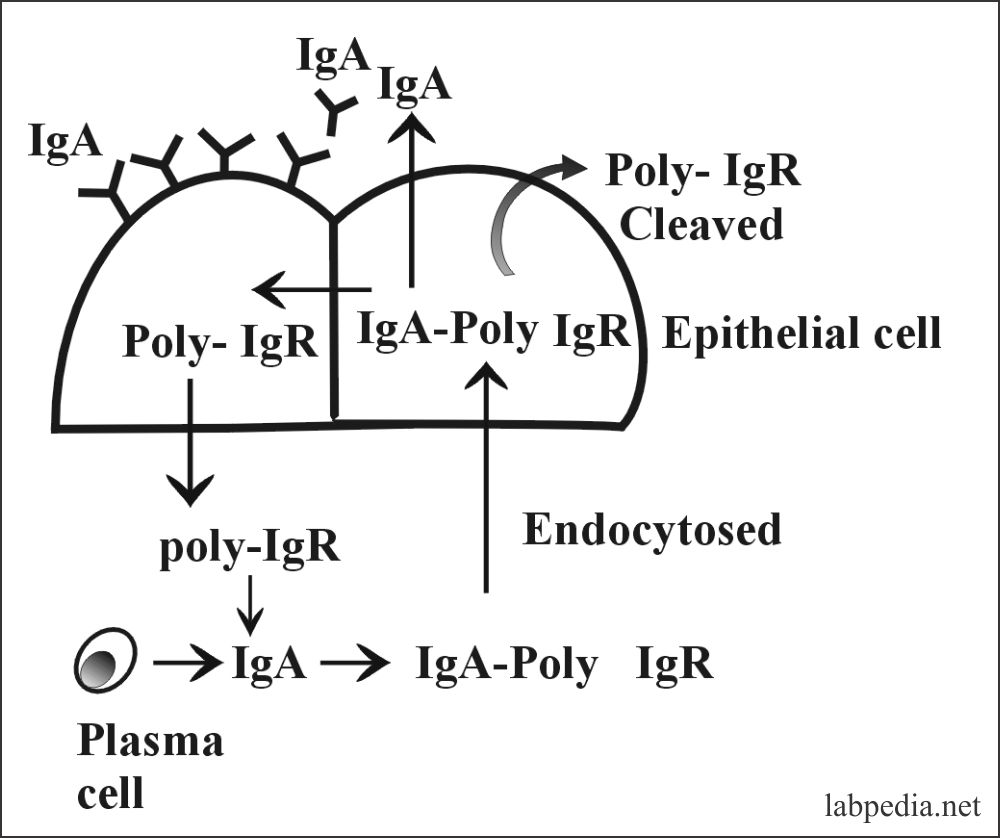
- The dimeric form is present in secretion and secreted with the help of poly-immunoglobulin receptor (IgA- poly-IgR) which is produced by the epithelial cells.
- Functions:
- The deficiency of IgA leads to infection of the gastrointestinal tract and respiratory tract.
- It has a major role in the protection of bacterial, viral, and protozoal infections of the mucosa by preventing their attachment and colonization.
- It can activate complement by the alternative path. It does not bind complement via the classical pathway.
- It is an effective opsonin, reacting with Fc α-Receptor on monocytes and neutrophils.
- It has antiviral activity.
- IgA secretory role:
- IgA is produced by the cells in the intestinal walls, from where it can directly go into the lumen or diffuse into the blood circulation.
- As the IgA is transported through intestinal epithelial cells or hepatocytes, it binds the glycoprotein called the secretory piece.
- This secretory piece protects the IgA from digestion by the gastrointestinal proteolytic enzymes and forms a complex molecule called secretory-IgA.
- This secretory-IgA protects the surfaces from invading microorganisms.
- It gives protection in the tears, saliva, nasal fluids, and colostrum.
- IgA monomer is present in high concentrations in human blood.
- At the end of the first years of life IgA level, 25% reaches the adult level. While 50% at 3.6 years of life. The average adult level is attained by 16 years of life.
- Cord blood IgA level = >1 mg/dL.
- CSF = 0.1 to 0.6 mg/dL.
- Adult = 90 to 450 mg/dL (55 to 270 IU/mL)
- The increased IgA level is seen in:
- Tuberculosis.
- Actinomycosis.
- Rheumatoid arthritis.
- Polyclonal gammopathy.
- Monocytic leukemia.
- IgA -myeloma.
- Chronic active hepatitis and Laennec’s cirrhosis.
IgA properties:
Present in Functions Increased in Decreased in - Gastrointestinal tract
- Respiratory tract
- Milk, colostrum
- Tears
- Saliva
- Genitourinary system
- Exocrine secretion
- Protect the mucus membrane from bacteria and the viruses
- Activates complement through an alternative pathway
- Protect mucus membranes from toxins, antibacterial agglutinins, ANA, and allergic reagin
- Chronic infections
- IgA myeloma
- Liver diseases
- Autoimmune diseases
- Wiskott- Aldrich syndrome
- Aggaglobulinemia
- Lymphocytic leukemia
- Malignancies
- Malabsorption syndrome
- Hypogammaglobulinemia
- Hereditary ataxia-telangiectasia
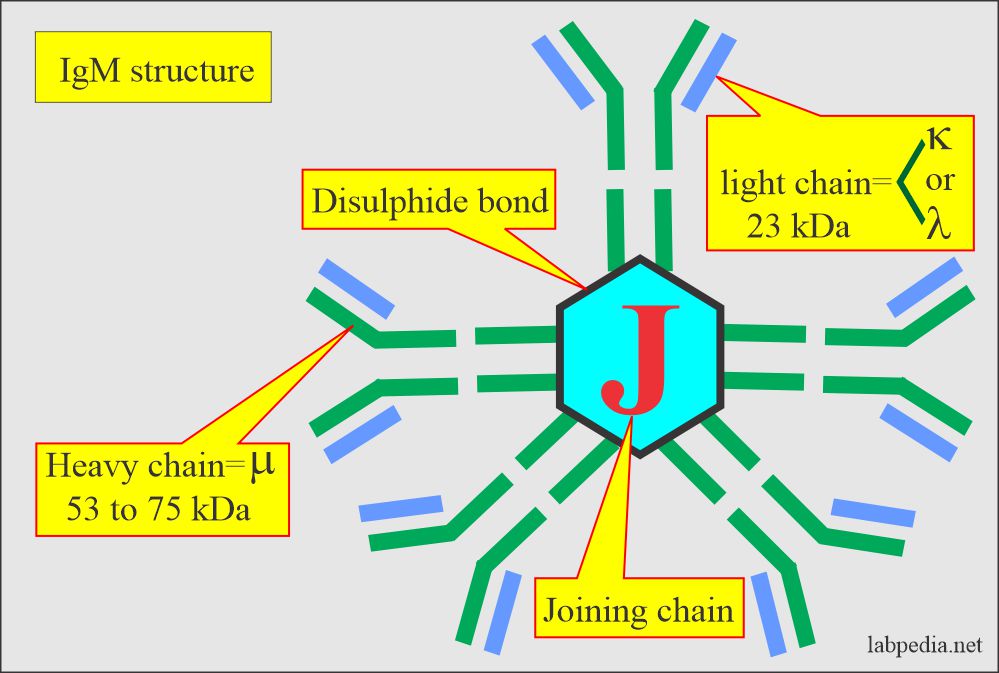
IgM
- IgM accounts for about 10% of the immunoglobulins. It is mostly confined to the intravascular space because of its large size.
IgM exists in two forms:
- IgM Monomeric form exists as a B-cell receptor for antigen. It has four domains in the heavy chain.
- IgM is a Pentameric form present in the serum, joined by J-chain, and functions as an antibody.
- Its molecular weight (5 molecules) is 900,000 and is 19S. The individual molecule has a molecular weight of 65000.
- Its concentration is approximately 120 mg/dl.
- IgM is the first antibody production in response to antigen by the B-L (so-called primary immune response).
- IgM as a pentamer is the most efficient activator of complement for the lytic reaction.
- It has 10 potential antigens binding sites.
- In fetal life, this is the immunoglobulin, which appears first. Its raised level in neonates indicates intrauterine infection.
- It has half of the life of 10 days.
- It is a poor toxin neutralizing Ab.
- It is a strong agglutinating antibody. It also takes parts in cytolytic reactions.
- The adult level is attained between 8 to 15 years.
- Normal male = 60 to 250 mg/dL (70 to 290 IU/mL).
- Female = 70 to 280 mg/dL (80 to 320 IU/mL).
- Cord blood = >20 mg/dL
- CSF is usually undetected.
- IgM is increased in:
- ute bSubacacterial endocarditis.
- Infectious mononucleosis.
- Trypanosomiasis.
- Actinomycosis.
- Malaria.
- Leprosy.
- Scleroderma.
- Polyclonal gammopathy.
- Waldenstrom’s macroglobulinemia.
IgM properties:
Present in Functions Increased in Decreased in Serum - Activate the complement system
- It is seen in the primary immune response
- It produced against gram-negative bacteria
- The rheumatoid factor is IgM
- It is produced in the ABO blood group system
- Waldenstrom’s macroglobulinemia
- Lymphoma
- Rubella infection in newborn
- Infectious mononucleosis
- Malaria
- Relapsing fever
- Trypanosomiasis
- Brucellosis
- Actinomycosis
- Amyloidosis
- IgG and IgA myeloma
- Agammaglobulinemia
- Lymphocytic leukemia
- Dysgammaglobulonemia
IgD
- It is found in very low concentrations in the blood.
- IgD exists as a monomer form with a molecular weight of 180,000 and is 7S.
- IgD has four domains in heavy chains.
- It is 0.2% of the total immunoglobulin of approximately 3-5mg/dl.
- There are isolated reports of its antibody activity (e.g. against insulin, penicillin, milk protein, diphtheria toxoid, nuclear antigen, and thyroid antigen.
- Its main function is not known but may be involved in the differentiation of B-lymphocytes.
- Its half-life is 2-3 days.
- It can not cross the placental barrier.
IgD properties:
Present in Functions Increased in - Cord blood
- Serum
- Unknown
- Help B-cell receptor
- IgD myeloma
- Chronic infections
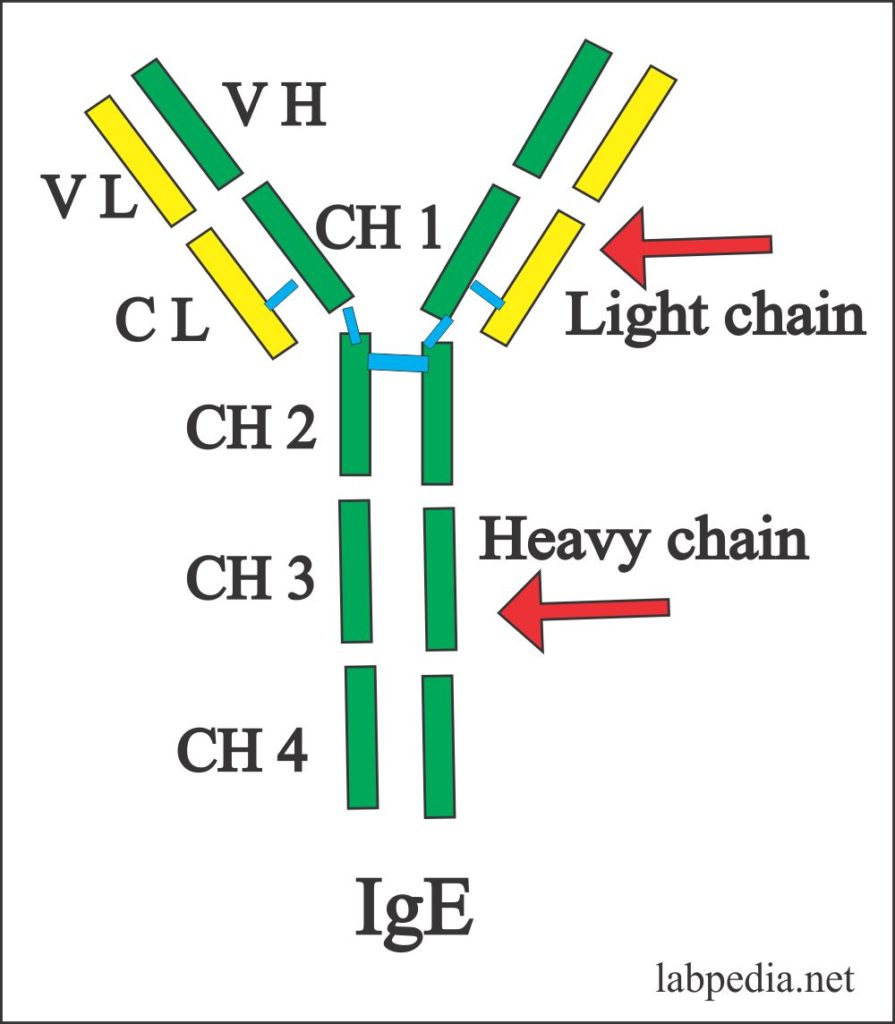
IgE
- It is present in traces in the blood of a person who is not exposed to the parasite.
- IgE exists as a monomer with a molecular weight of 190,000 and is 8S.
- It is 0.004 % of total immunoglobulin.
- It is the largest Ig monomer, because of the presence of four CH domains.
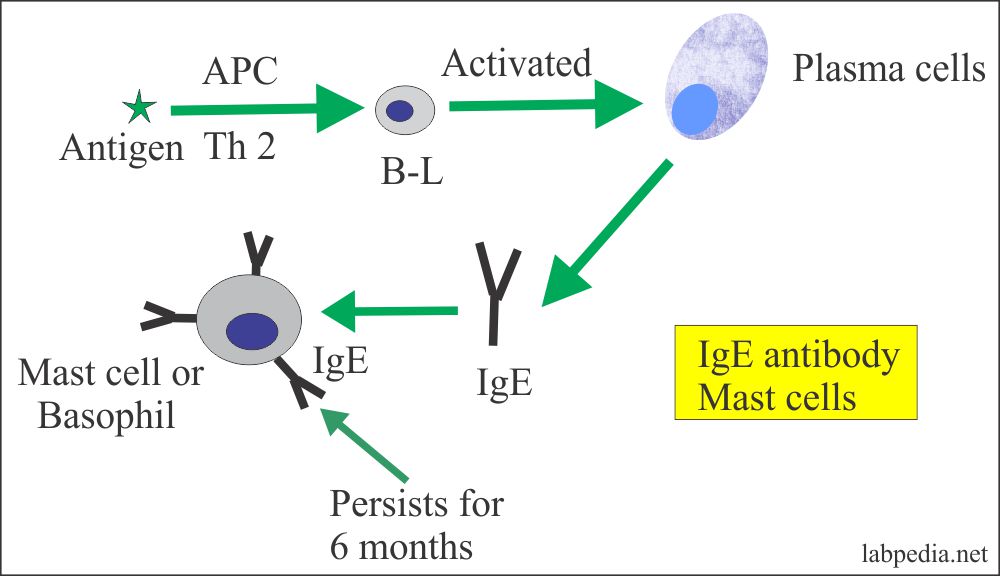
- IgE is associated with type-I hypersensitivity reactions (allergic reactions).
- This will lead to an anaphylactic reaction.
- It is unable to activate complement via the classical pathway.
- It is important in immunity for parasitic infestation.
- It is present in the serum of a healthy person is the extremely low concentration (150 ng/ml in nonatopic person)
- It binds to FcεRI which is a high-affinity receptor present on mast cells and Basophils.
- It will lead to the release of histamines and heparin from the mast and basophil cells.
- Low-affinity Fc ε RII (CD23) is present on B-lymphocytes and Eosinophils.
- It cannot cross the placental barrier.
IgE properties:
Present in Functions Increased in Decreased in - Serum
- Interstitial fluid
- Anaphylaxis (allergic reaction)
- Type hypersensitivity reaction
- Protects against parasitic infestation
- Asthma
- Hay fever
- IgE myeloma
- Atopic dermatitis
- Anaphylactic reaction
- Congenital Agammaglobulinemia
Summary of Immunoglobulin characteristic features:
| Properties |
H-Chain
|
L-Chain | Sub-class | Agglutination | Precipitation | Lysis | Neutralization | Opsonization | C-activation | Concentra tion | ||
| IgG |
7S 75% Monomer Late Ab |
γ |
Κ or λ |
IgG1 IgG2 IgG3 IgG4 |
W |
S |
W |
Virus | Toxin |
+++ |
S ++ |
900-1800 mg/dl |
|
++ ++ |
++ + |
|||||||||||
| IgA |
15% 7,9,11,135 Monomer & dimmer, surface protection |
α |
Κ or λ |
α1α2
|
+ |
+ – |
– |
++ + |
– |
+ |
Neg. |
154-294 mg/dl |
| IgM |
10% Monomer & pentamer 19 S |
μ |
Κ or λ |
μ 1 μ 2 |
S |
+ |
S |
+ |
– |
+ |
S +++ |
67-145 mg/dl |
| IgE |
Monomer 8 S, reagin |
ε | Κ or λ | – | – | – | S | – | – | – | – | 0-0.5mg/dl |
| IgD |
Monomer 7 S |
δ | Κ or λ | – | – | – | – | – | – | – | – | 3-5mg/dl |
Summary of Immunoglobulin characteristic features:
| Half-life | Site | Mol. Wt | Placental Transfer | Activation of the alternative complement | Distribution |
Present in Milk |
|
| IgG | 23 Days | LN & spleen | 160,000 | +++ | – | Equal I/V Extravascular | ++ |
| IgA | 6 Days | Mucosal surfaces |
170,000 to 385,000 |
– | + | Intravascular & secretions | ++ |
| IgM | 5 Days | Lymph node & spleen | 900,000 | – | – |
Mostly I/V And BCR |
Traces |
| IgE | 1-5 Days | Lymph node & spleen | 190,000 | – | – | Mostly I/V cell surface | – |
| IgD | 2-8 Days | Lymph node & spleen | 180,000 | – | – |
Present on B-L surface |
– |
W = Weak S = Strong
CLASS AND SUBCLASS SWITCHING (ISO-SWITCHING)
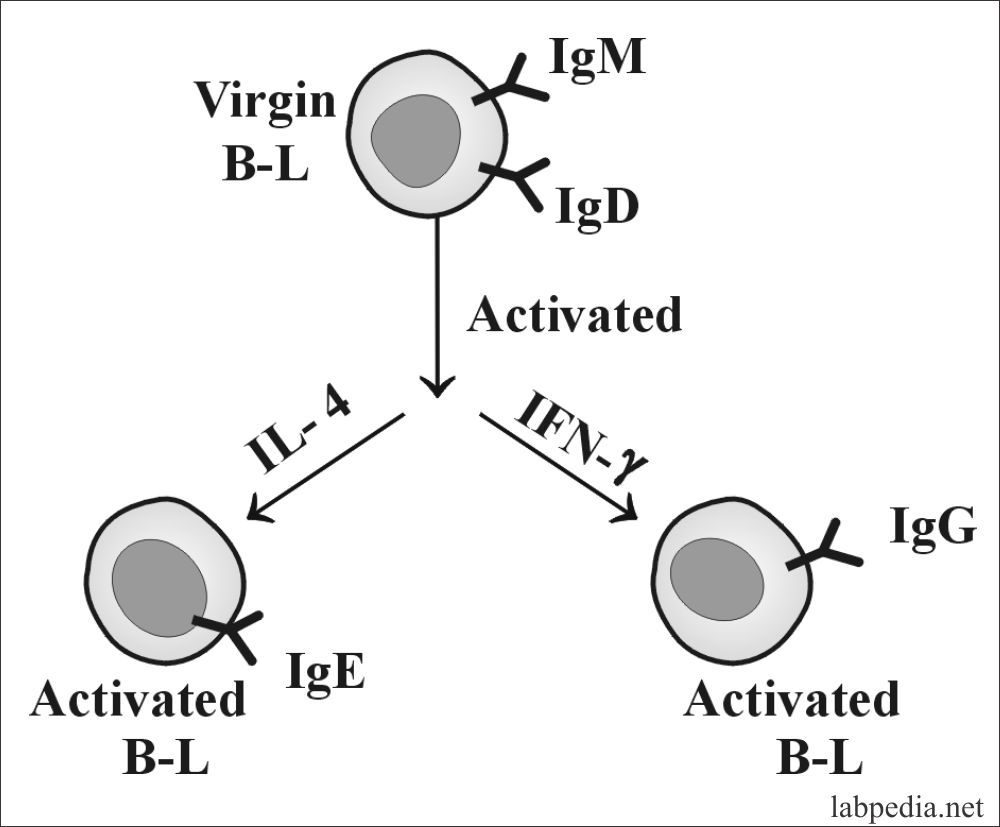
Class switching involves the solution during B-lymphocyte’s development of different immunoglobulin CH genes. The site-specific recombination process deletes most of the DNA between the VH region, the desired CH region. So the majority of class switching occurs by DNA recombination, and some part is at the RNA level. There is a stretch of repetitive DNA known as the switch region. Switching occurs by recombination between the switch signals with the deletion of the intervening DNA. The initial switching event takes place from the switch region, switching to other isotypes can take place.
In vitro culture studies are important. Culture of B-cells with polyclonal activator alone, produce IgM in the culture fluid. The addition of IL-4 induces switching to IgE production, while the addition of IFN-g induces production of IgG and no IgE.
The other factors that clearly must have an effect on class switching are the nature of the antigen.