Chapter 30: Serological Tests and Its Basis
Serological Tests
Definition
Serology is an in-vitro study of serum where we see antigen and antibody reactions.
Serological tests are used for:
- Diagnosis of the diseases.
- It helps in treatments.
- Guide for the prognosis of the disease.
- Antigens and antibodies can be quantitated.
Serological tests may be:
- Specific for diagnosing viral and bacterial infection, e.g., a Widal test for Enteric fever and a Brucellosis test.
- Non- specific tests give evidence for some diseases like Syphilis and SLE.
Serological tests can be:
- Qualitative.
- Quantitative.
The main use of the Serological Test
- Identification of microorganisms.
- Detection of antibody.
- Detection of autoantibody.
- Detection of offending drugs.
- Immunoassay of hormones. These serological means can measure the minute amount of hormones.
- Detection of immunologic abnormalities.
Disadvantages of Serological Tests
- These tests have little value in the early phase of the disease, that is 7-10 days. Usually, these tests are positive after 10 days.
- Changing titer (rising) is more important for confirmation of the disease. If the titer is low and there is no rising (change) titer after 5-7 days, this test has no value.
Samples for Serological Tests
- The best sample is serum.
- Other fluids, which can be used, are:
- Plasma
- CSF
- Fluids
TYPES OF SEROLOGICAL TESTS
These serological tests are based on antigen and antibody reactions. These are:
- Agglutination
- Precipitation
- Flocculation-modification of precipitation
- Fluorescent antibody test
- ELISA (Enzyme-linked immuno-absorbed assay)
- Radioimmunoassay (RIA)
- Electrophoresis
- Immunoelectrophoresis
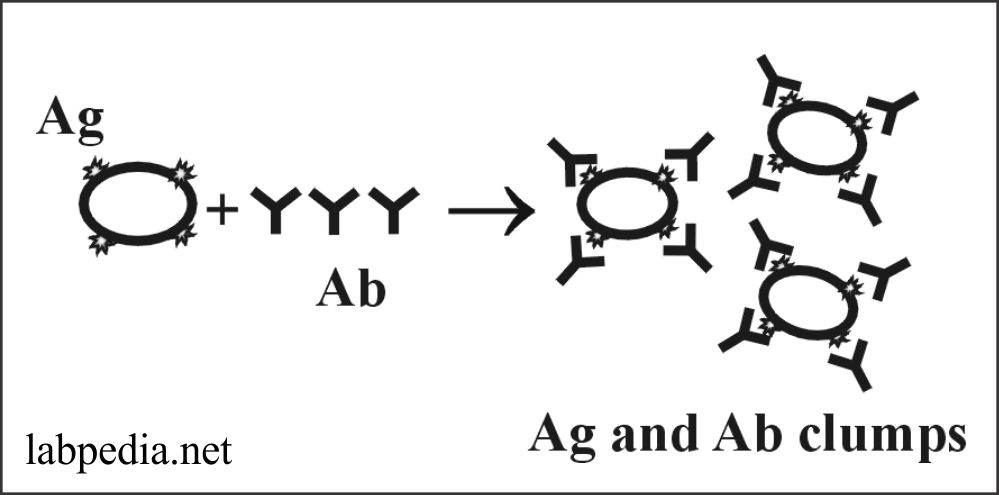
Agglutination
Principle:
The antigens are in a particulate form where antigen and antibody form clumps. This is a direct measurement of antibody binding to antigen. It is used in the quantitative serologic assay. Antibodies are called agglutinin.
The antigen may be present in the bacterium, which forms an agglutinate with the antibody. At the same time, antigen on RBC forms haemagglutination.
The antigen may be bound to RBC, and antibodies may be detected; this is called passive haemagglutination.
Uses
- For ABO blood grouping.
- Widal test for diagnosis of enteric fever etc.
Precipitation
Principle:
Ag & Ab’s reaction is just like agglutination with the difference that here antigens are insoluble form. The antibodies giving precipitation are called precipitation.
Precipitation may be seen:
- In liquid media.
- In semisolid media.
Precipitation in Liquid Medium
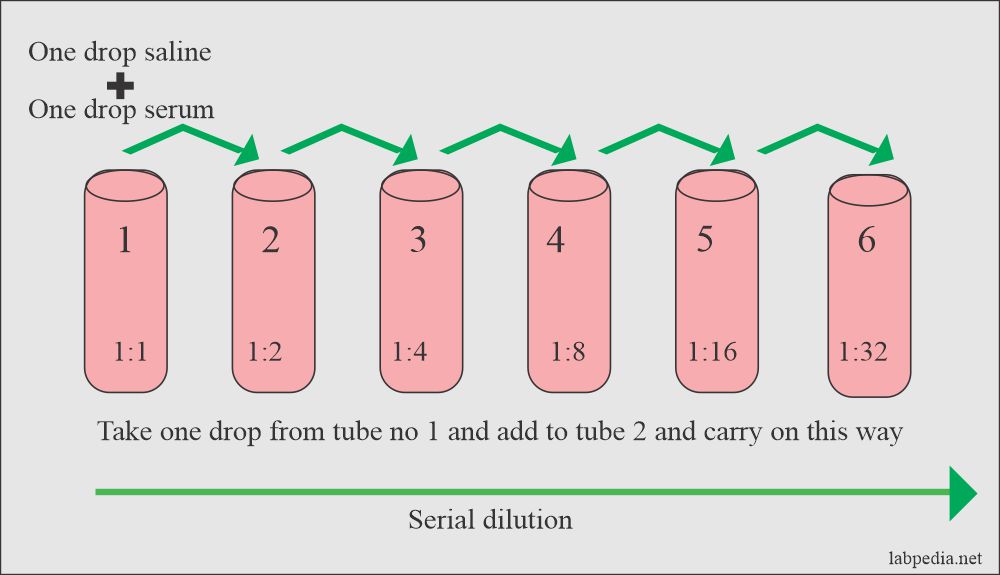
Antigens and antibodies are present in liquid form and precipitate depending upon the concentration of antigens and antibodies. The antibody can be quantitated by fixing the number of antigens and with a serial dilution of serum (Ab).
Procedure: Serial dilution is shown diagrammatically.
- Add Antigen to tubes.
- Add serum 0.1ml and 0.3ml normal saline (1:4 dilutions).
- Add 0.1ml of normal saline to all other tubes.
- Now take 0.1ml from tube 1 and add to tube 2.
- Then take 0.1ml from Tube 2 to tube 3. In these ways, there will be serial dilution.
- Now the last tube showing a small amount of precipitate is the titer. e.g., in this case, 1:32.
Precipitation in Semisolid Media
This is done in Gel (agar), and this method is called Ochterlony Gel diffusion, which was described by Swedish scientists. This can be used as:
- Qualitative or
- Quantitative
This may be done as:
- Single radial immunodiffusion(SRID)
- Double radial immunodiffusion (DRID)
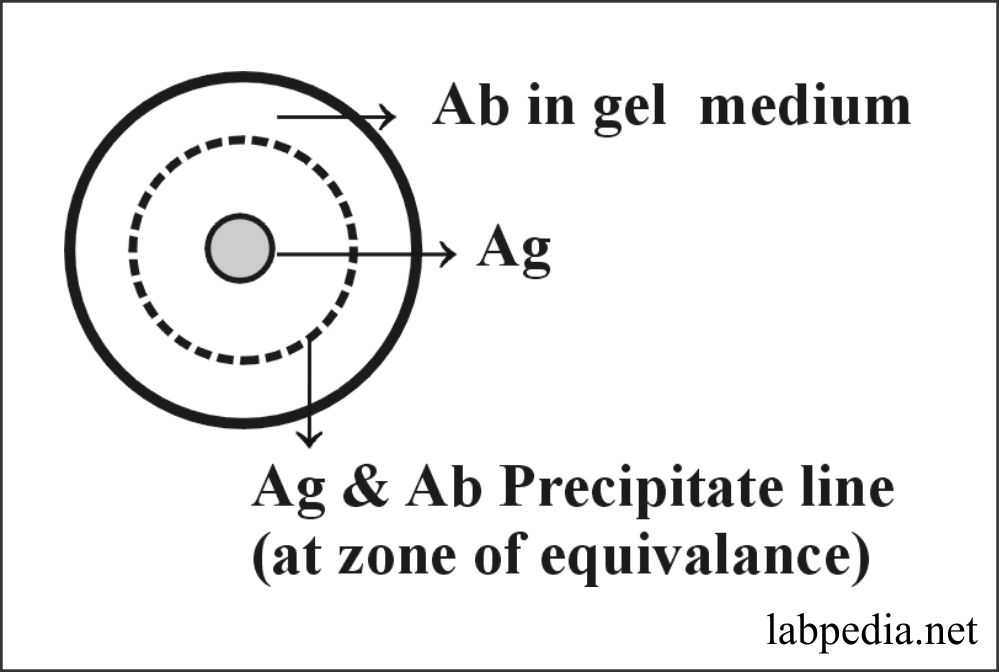
Single Radial Immunodiffusion (SRID)
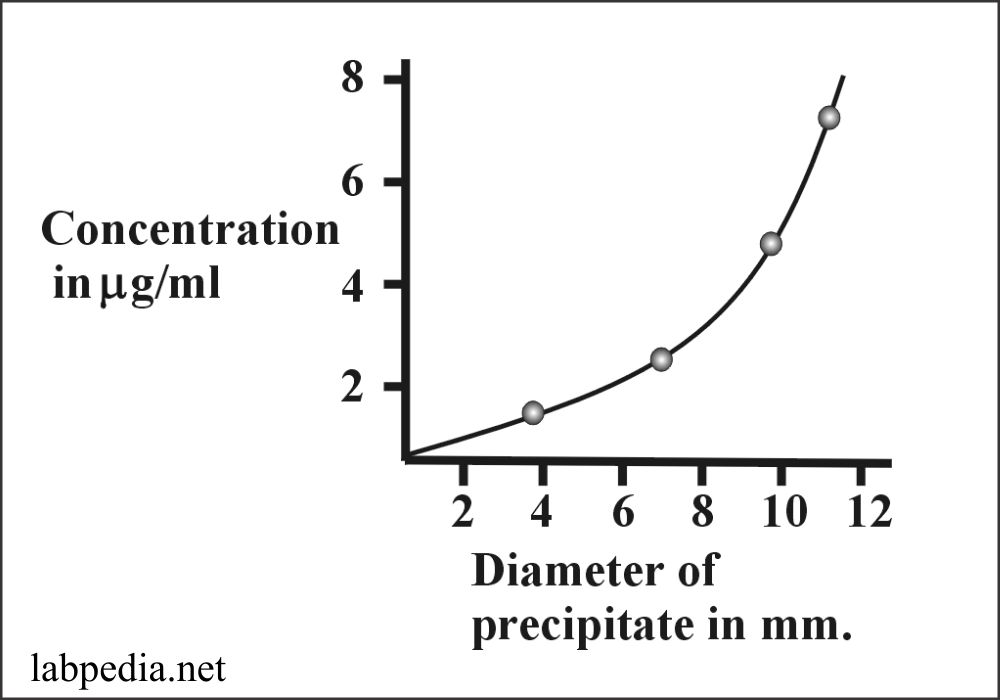
This method can measure up to 1-3μg/ml. In this method, the antigen is placed in Gel-plate’s central well, and antibodies are mixed in the Gel. Now antigen diffuses in the medium and gives rise to a line of visible precipitation by combining Antigen and Antibody where they meet at the zone of equivalence.
Now a plot can be graphed by doing this procedure with a known antibody concentration.
Double Radial Immunodiffusion (DRID)
The gel medium does not contain any antigen or antibody in this method. Three wells are made, where two antigens are placed in two wells, and in the third well, the antibody is added. Now Ag & Ab diffuse out in the gel-medium and give rise to a precipitate line at the zone of equivalence.
Fluorescent Antibody Technique
Coons described this in 1940.
Principle:
Antibodies are labeled by fluorescent material, then antigen is added. These are washed and seen with the help of a fluorescence microscope. No fluorescence will be seen if the antibody is not bound to the antigen.
Stain materials used are:
- Rhodamine
- Auramine
This method may be:
- Direct
- Indirect
- Sandwich
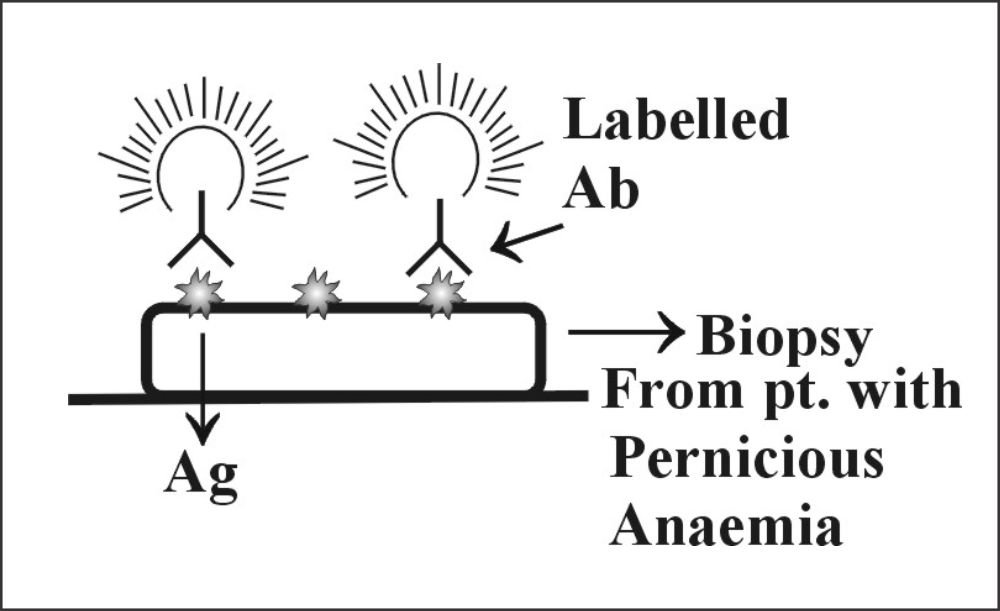
Direct Fluorescent method
The biopsy material is taken on the slide, which contains antigen. Now add labeled antibody (), and after incubation, wash the slide. Now see under the fluorescent microscope; in positive cases, fluorescence will be seen.
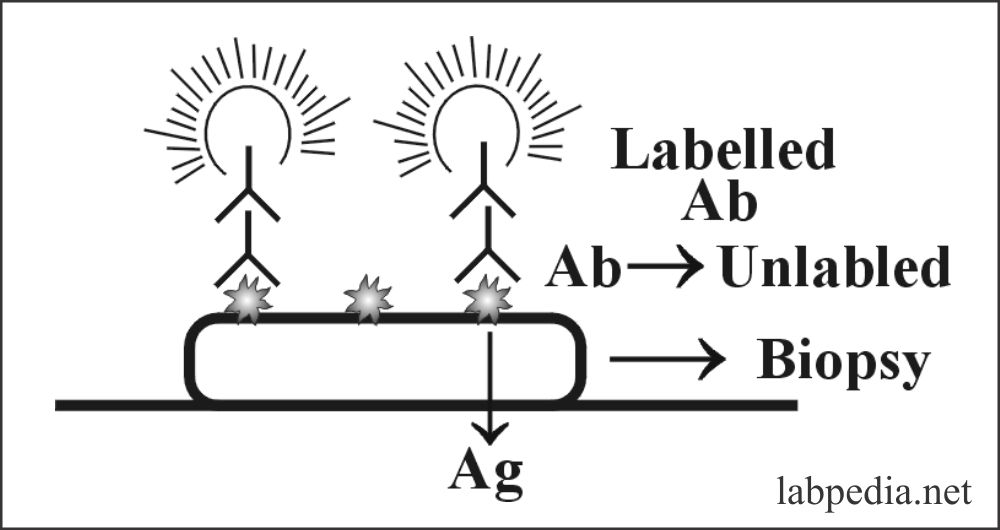
Indirect Fluorescent Antibody
In this case, there is a double layer of antibodies. One is labeled, and the other is unlabelled. The first biopsy (antigen) is treated with an unlabelled antibody; then, the slide is washed. Now add labeled antibody, which will attach with antigen + antibody complex, and fluorescence will be seen.
Advantages of Indirect F.A.
- There is better fluorescence.
- This test is more sensitive.
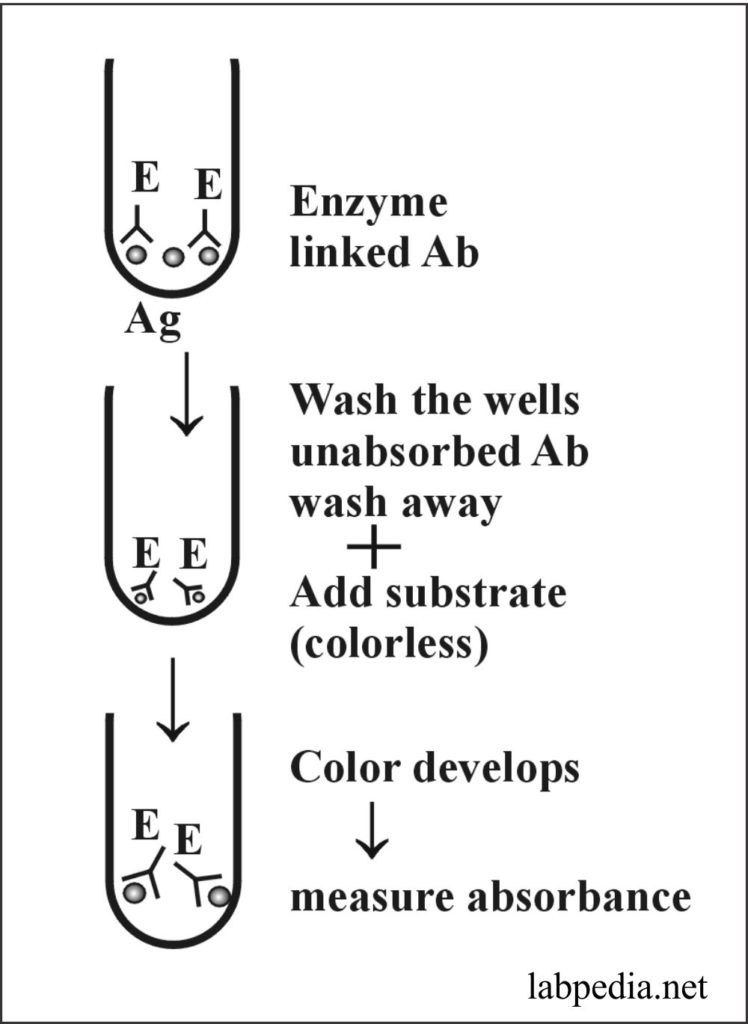
ELISA(Enzyme-linked Immuno Absorbent Assay)
Principle:
ELISA relies upon the “capture” antibody fixed to the plastic plate. Antigen or antibody-coated beads or wells are taken. Suppose there are antigen-coated wells. Now add serum of the patient after washing and add enzyme-linked antibody to these wells; after incubation, washes the wells. Now add substrate, and color develops. Stop the reaction and read by photometer, which will measure absorbance according to the intensity of color, depending on the number of antibodies bound to the antigen.
The enzyme used is Peroxidase and alkaline phosphatase.
This procedure can now be modified by fixing the antibody to pallets and adding antigens with an enzyme.
Advantages
- These are very sensitive and safe.
- There is no radiation hazard.
- These are economical and simple instruments are needed.
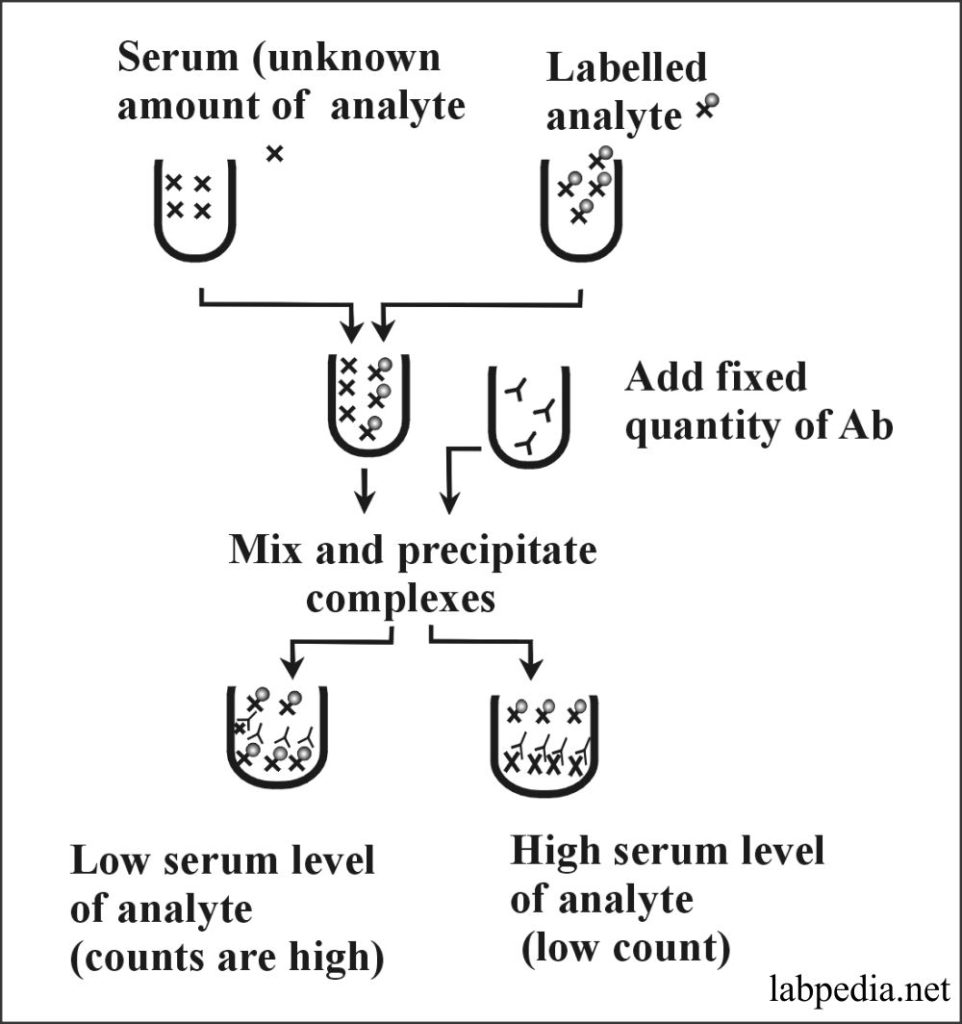
Radioimmunoassay (RIA)
Principle:
RIA is used to detect molecules (analytes) in circulation. It depends upon the availability of an antibody that specifically recognizes the analyte. This is basically a competitive assay. A fixed amount of antibodies is added and compete for the analyte, either in the sample or added to the sample in a radiolabeled form (e.g., bound to I125). Analyte-antibody complexes form and are precipitated by physicochemical means. Gamma-Counter measures the radioactivity in the precipitate.
A high radioactivity level reflects the low concentration of an analyte in the serum sample, while a low radioactive count indicates a high level of an analyte.
RIA methods are also used to measure small quantities like hormones, even in pictogram levels.
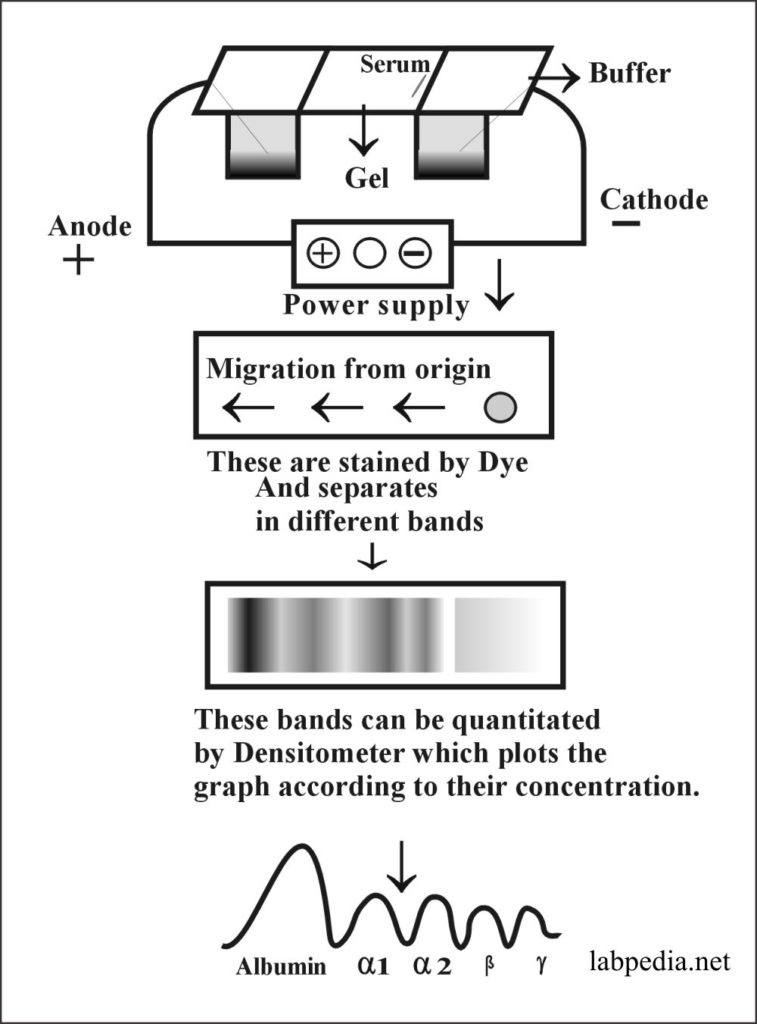
Electrophoresis
Principle:
This method is used to separate proteins in serum in the electrical field. Also used to separate different types of Haemoglobins. Proteins migrate within the electrical field according to their charge. In turn, this migration is influenced by pH and the optimum separation achieved by Buffer at pH 8.6.
Once separated on gel or fitter, these are stained with dye.
Albumin is the major constituent of serum proteins and at this pH migrates fast as a negatively charged molecule towards the anode.
Electrophoresis is used for:
- To find hypergammaglobulinemia.
- To find the oligoclonal band in myelosclerosis.
- Abnormality in other proteins.
- It can be used for hemoglobinopathy.
Serum protein can be quantitated in the densitometer, which plots the graph according to each protein’s concentration.
Immunoelectrophoresis
For the separation of immunoglobulin, modification of electrophoresis is used as immunoelectrophoresis.

Very nice recognition of information
how you can send this notes for self evaluation and study
I have added a few questions. I think you need to read a few books.