Drabkin’s Solution for Hemoglobin, Preparation of Drabkin’s Solution
Drabkin’s Solution for Hemoglobin Estimation
- There are commercially available kits that make it easy to prepare the solution.
- When blood is analyzed in the hematology analyzer, these instruments also estimate hemoglobin levels.
What sample is needed for hemoglobin estimation?
- Whole blood is needed for hemoglobin estimation.
What precautions will you take to keep the Drabkin’s solution?
- Store the solution in a dark-colored bottle and keep it in a dark location to protect it from light.
- If the solution is cloudy after adding the blood, centrifuge before the reading; this may be due to nonhemolyzed RBCs or globulins.
- Spectrophotometer cells should be fingerprint-free; otherwise, the reading will be high.
- Drabkin’s solution is a pale yellow, clear fluid; it should not be used when it is cloudy.
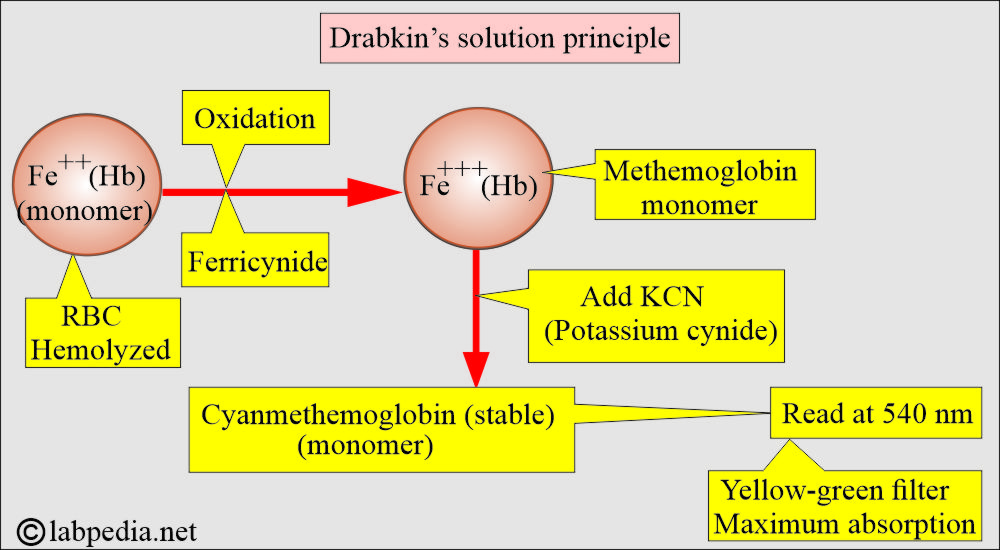
What is the principle of Drabkin’s solution?
- Whole blood is diluted 1:200 with Drabkin’s solution, which contains potassium ferricyanide and potassium cyanide.
- RBCs are hemolyzed by Drabkin’s solution.
- Now hemoglobin is oxidized, and its derivatives, except sulfhemoglobin, form methemoglobin in the presence of alkaline K-ferricyanide.
- The methemoglobin reacts with K-cyanide to form a very stable compound, cyanmethemoglobin, and this complex has maximum absorption at 540 nm.
- Chain of chemical reactions:
- Potassium ferrocyanide converts hemoglobin to methemoglobin.
- Potassium cyanide converts methemoglobin into stable cyanmethemoglobin.
- Potassium dihydrogen phosphate acts as a buffer.
- Non-ionic detergents cause lysis of the red blood cells.
How will you manually make Drabkin’s solution?
- Hemoglobin (Drabkin’s) solution can be prepared in the laboratory.
- Drabkin’s solution reagents needed are:
- Potassium ferricyanide = 200 mg
- Potassium cyanide = 50 mg
- Potassium dihydrogen phosphate = 140 mg
- Non-ionic detergent = 1 ml
- Distal water = Make up to 1000 ml (1 L)
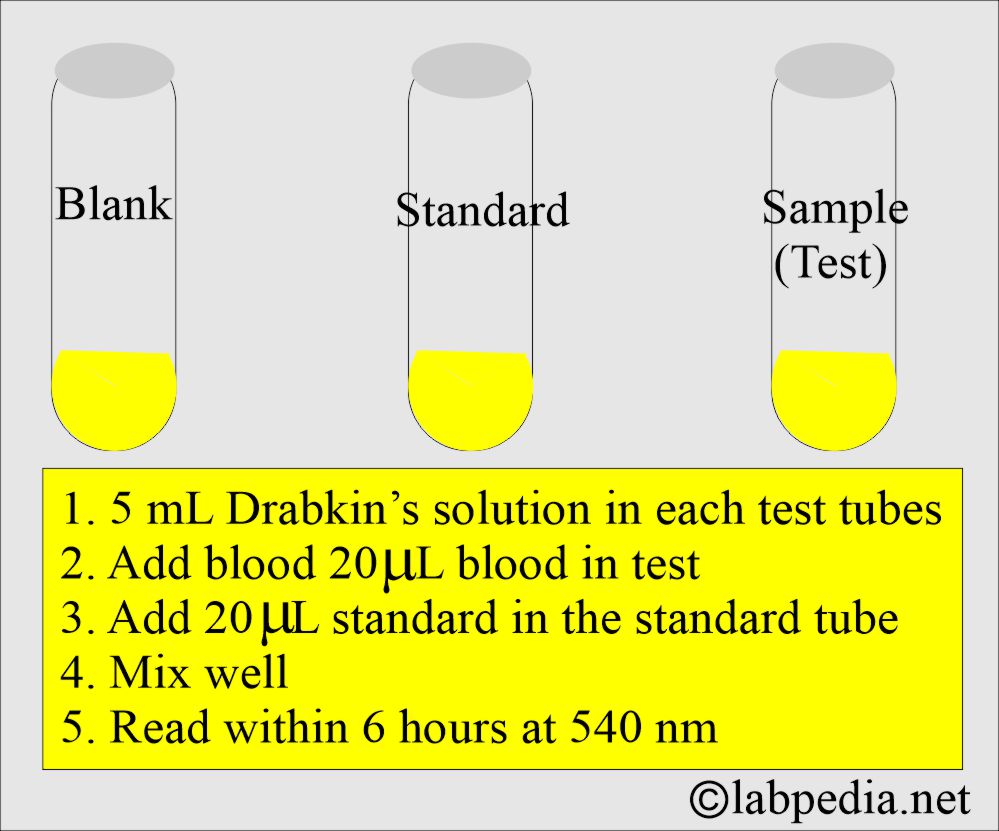
What is the procedure for estimating hemoglobin by Drabkin’s solution?
- Take 20 microliters of blood and add 4 mL of Drabkin’s reagent = 1:200 dilution.
- Or take 20 microliters of blood and add Drabkin’s reagent, 5 mL, to achieve a 1:250 dilution.
- Now mix well.
- Read within 6 hours of mixing the blood with Drabkin’s solution.
- Read on a spectrophotometer at filter 540.
- Read against the blank of Drabkin’s solution (Drabkin’s solution can be used as a blank).
- Also, read the standard solution (12 G/dL) with the same dilution as the test sample.
- Read by the spectrophotometer; the reading is called optical density (OD).
What is the stability of Drabkin’s solution?
- It is stable for one year at room temperature.
- Always keep in an amber-colored bottle.
What are the normal hemoglobin levels?
- Adult male = 14 to 18 g/dL
- Adult female = 12 to 16 g/dL
- 10 years old child = 12 to 14.5 g/dL
- 3 months old infants = 9 to 14 g/dL
- Newborn = 17 to 23 g/dL
What are the physiological variations of Hemoglobin (Hb)?
- Strenuous physical exercise.
- There is a diurnal variation, with the highest level in the morning and the lowest in the evening.
- High altitude increases the Hb concentration.
What are the false causes of raised hemoglobin (Hb)?
- Hemoconcentration due to dehydration and burns.
- Immediately after hemorrhage.
- If taken during the I/V infusion, it contains iron.
What are the advantages of Drabkin’s solution?
- It produces a stable structure with all types of hemoglobin.
- Except sulfhemoglobin.
- It gives an accurate reading with the spectrophotometric method.
What are the disadvantages of Drabkin’s solution?
- Cyanide is a carcinogenic chemical.
- Handle with care.
- Avoid inhalation or ingestion.
Questions and answers:
Question 1: What is the end result of the KCN reaction for hemoglobin?
Question 2: What is the physiological variation in the hemoglobin?



You shows the principle of drabkin solution but not show how we are apply this solution
Dear
As I have understood from your question, that you want the procedure by Drabkin’s solution. It is already given there. Or please explain your question.
I got good news
ok Thanks.
Can we take 2ml reagent and multiple the reading with 20
You have to keep the same dilution, that is very important.
Sir how to measure bilirubin ??
Dear nowadays nobody make reagents themselves. Ready-made kits are available in the market. Definitely you need a lab facility.
what is the function of potassium dihydrogen phosphate in drabkin’s solution ?
This has the buffering action.
Can drabkins reaction work with freeze dried hemoglobin instead?
I think if you thaw the frozen Hb, then try with control. That may work.
What’s the quality control of drabkin solution?
You can run known control of hemoglobin. These known controls are available from various companies.
what is the function of the non-ionic detergent in the drabkins solution ?
Please see the following link for your question.
https://patents.google.com/patent/US4341527A/en
May i ask sir, i read that a source of error when using the Drabkin’s reagent was a possible increase of globulins which can then be fixed using the dihydrogen potassium phosphate – is this true? and if so, how come what’s the principle behind it? Thank you !!
I have given diagrammatically the principle of Drabkin’s solution.
https://labpedia.net/haemoglobin-part-3-hemoglobin-solution-drabkins-solution-preparation/
Can we prepare the standard solution of cyanmethemoglobin process in lab…if it is not available commercially?
You can prepare Drabkin’s solution. I have described the procedure.
sir, what is OD in formula?
OD is the reading by the spectrophotometer, called optical density. Read the article again.
https://labpedia.net/drabkins-solution-for-hemoglobin-estimation/
Sir is Hb levels of 17.4 normal according to Drabkins method sir?
This is a higher limit. Please keep on checking Hemoglobin to rule out the possibility of polycythemia. It would be better to consult a hematologist.
Sir
Can hemoglobin react with potassium thiocyanate? Please
You can use sodium cyanide instead of potassium cyanide.
The procedure for commercially available cyanmethemoglobin standard soln is differ from the above mentioned one…
because if we use commercially available std. soln we should use directly if the soln. is in RT.
You can use a ready-made solution.
Hi, thank you for this useful publication. If I want to measure hemoglobin from plasma that was mixed in Drabkin reagent, would it be suitable to produce a standard by mixing a known quantity of lyophilized hemoglobin in Drabkin reagent? This way, I could build a standard curve with increasing hemoglobin concentrations, am I right?
https://labpedia.net/laboratory-part-1-serum-plasma-preparation-specimen-storage-and-precautions/
Please see this topic and it will answer your question.
https://labpedia.net/laboratory-part-1-serum-plasma-preparation-specimen-storage-and-precautions/
Please see this topic, and it will answer your question.
How to discard Drabkin’s reagent after use
Section 13. Disposal Considerations
In the fume hood, add the Cyanide solution to a solution of 1% Sodium Hydroxide (about 50 mL/g of Cyanide). Household bleach (about 70 mL/g of Cyanide) is
slowly added to the basic Cyanide solution with stirring. When the addition of the bleach is complete, the solution can be tested for the presence of Cyanide
using the Prussian Blue test: to 1 mL of the solution to be tested add 2 drops of freshly prepared 5% aqueous Ferrous Sulfate solution. Boil this mixture for at
least 60 seconds, cool to room temperature, and then add 2 drops of 1% Ferric Chloride solution. The resulting mixture is made acid to litmus with 6 Molar
Hydrochloric Acid (prepared with equal amounts of concentrated Hydrochloric Acid and water ). If Cyanide is present, a deep blue precipitate will form.
(Concentrations of greater than 1 ppm Cyanide can be detected.) If the test is positive, more bleach is added to the Cyanide solution, and the test is repeated.
Continue until no Prussian Blue precipitate is formed. Wash the solution down the drain with excess water. Always dispose of in accordance with local, state
and federal regulations.
Section 14. Transport Information
https://us.vwr.com/assetsvc/asset/en_US/id/8452666/contents
Please read this article.
What can be used instead of potassium dihydrogen phosphate?
Please read this article, and it may help you.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC477205/pdf/jclinpath00098-0110b.pdf
I want to try using actual whole blood with the above method.
Do I need to process the blood in any way before using it?
Can I use chemistry analyser since we have blank, standard and test
Your question is not clear to me. Please explain your question.
How do I make the standard? or where can I purchase the standard?
You can collect serum of different patients. Mix all sera and check chemicals like glucose, blood urea etc. Keep the record. Now make aliquots and freeze those. This is the internal control system. Run daily one of the aliquots. Compare with the first noted readings.
For external quality control or standards , you can buy from Roch or Abbott company.
Hello Dr.,
Can we use this procedure for aquatic fishes?.
Thanks in advance.
I think if you are doing biopsy material staining, it will work.
what is the reference for reading the results within 6 hours after mixing?
You have to run the standard. Also, you can make your own reference values. You can get known controls from various companies.
I’m sorry I didn’t make my question clear. I mean what reference was used to make that statement “run within 6 hours after mixing” ?
I’m asking because my professor questioned this on a lecture I made using your article