Blood sample Types, Anticoagulants, Preservatives, Adverse effects of Additives
Blood sample Types
Indications for the whole blood, plasma, and serum:
- A whole blood sample is used for blood gases and ammonia.
- It may be used for glucose, urea nitrogen, and lactate estimation.
- Serum and plasma are used for the majority of the chemical tests.
- The disadvantage of plasma is that there are chances to form fibrin clots if you store the sample.
- These microclots may block the probe of the analyzer.
- Plasma is not a good sample for electrophoresis.
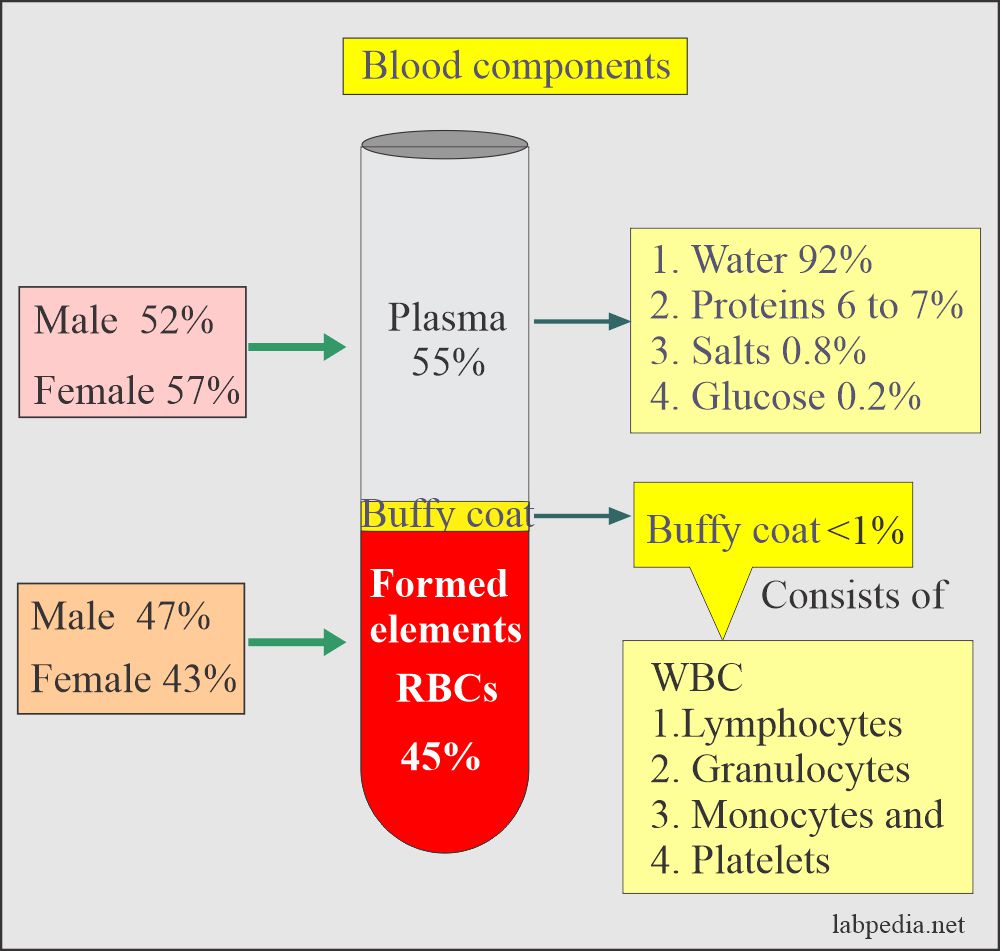
Definition of the blood:
- Blood consists of formed elements (RBCs, WBCs, Platelets) in a liquid portion called plasma.
- There is a difference in the plasma and the serum for estimating various substances in the blood.
Type of patients for the blood samples:
- Pediatric patients:
- If this is the first time sample from the child, then gain his confidence.
- Blood for neonatal screening is collected to rule out hypothyroidism, phenylketonuria, galactosemia, and hemoglobinopathies.
- For phenylketonuria: The baby must have received 3 to 4 days of full milk, and the infant has taken the feed.
- Adult patients:
- Be friendly and explain the procedure.
- Patients in the ICU:
- Patients are unconscious. The patients are unconscious, but still, there may be a need to take blood samples.
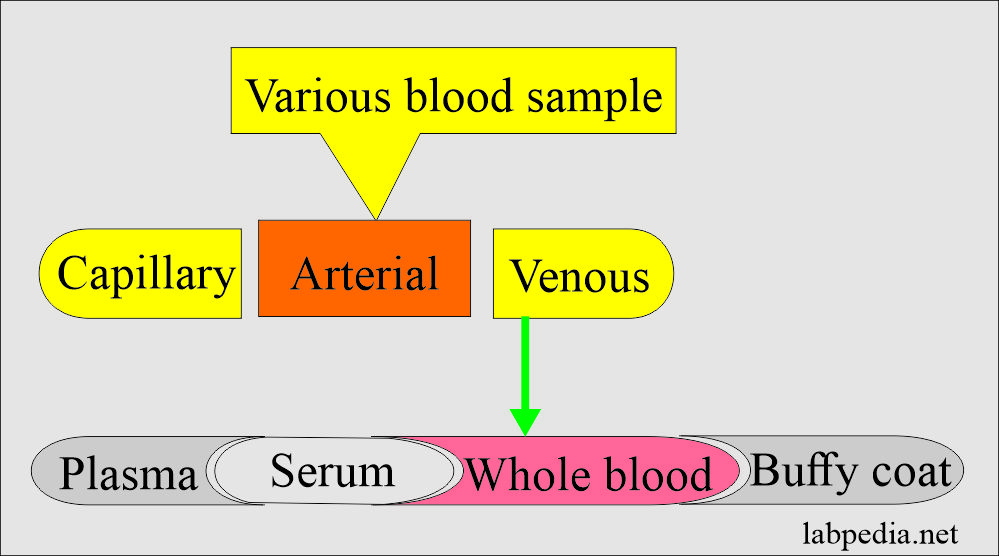
Types of blood samples:
- Various types of blood are:
- Arterial blood.
- This is the best sample for studying blood gases (O2).
- Venous blood.
- It differs from arterial blood and has lower substances than arterial blood.
- It has a higher concentration of body waste products like CO2, organic acids, and ammonia.
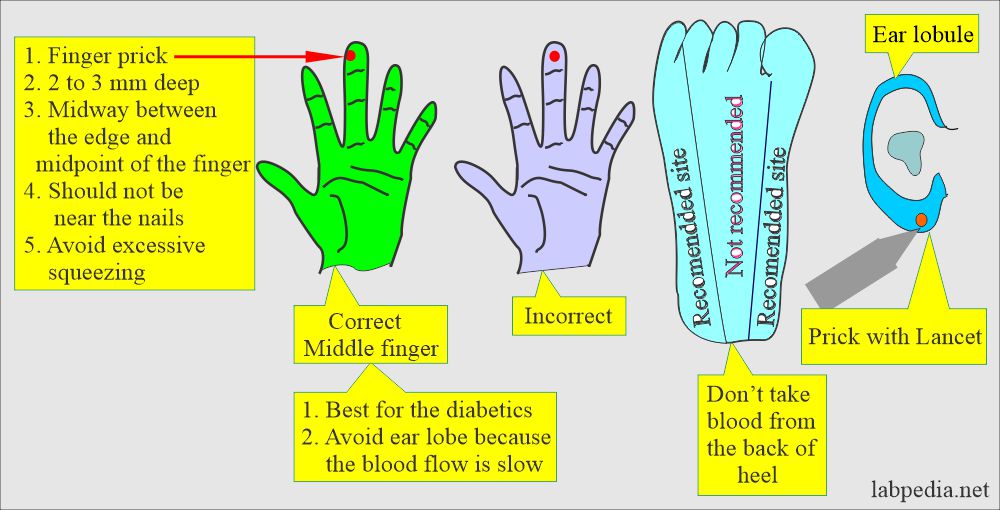
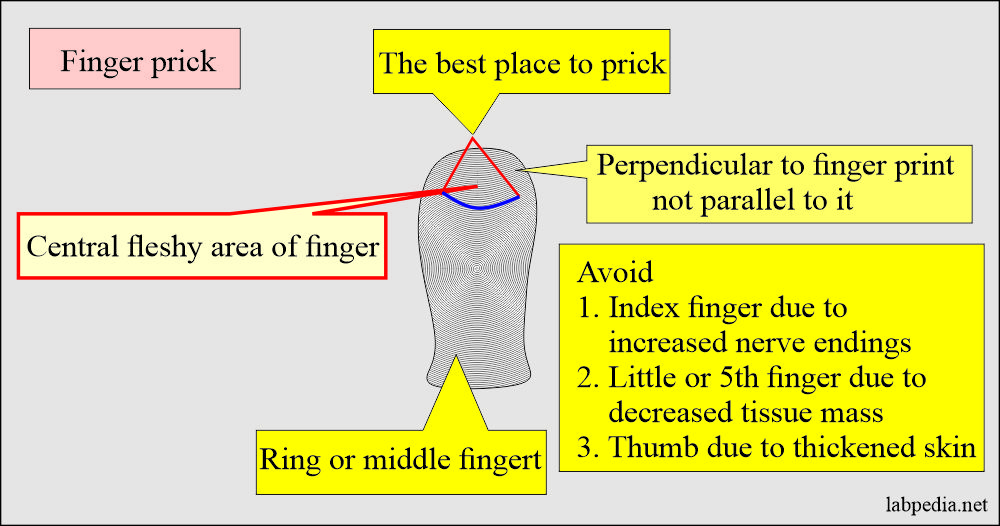
- Capillary blood (skin puncture).
- This is good for a small quantity of blood.
- This is near to arterial blood than venous blood.
- The patient in shock has 50% less blood glucose than venous blood.
- Warm the finger from where you are taking the blood sample.
- The heel is the best site for a newborn under 3 months to get a small blood quantity.
- The depth should not be >2.4 mm on the heel.
- Avoid the central portion and back of the heel.
Venous blood (venipuncture) sample:
- For larger quantities, we will take venous blood.
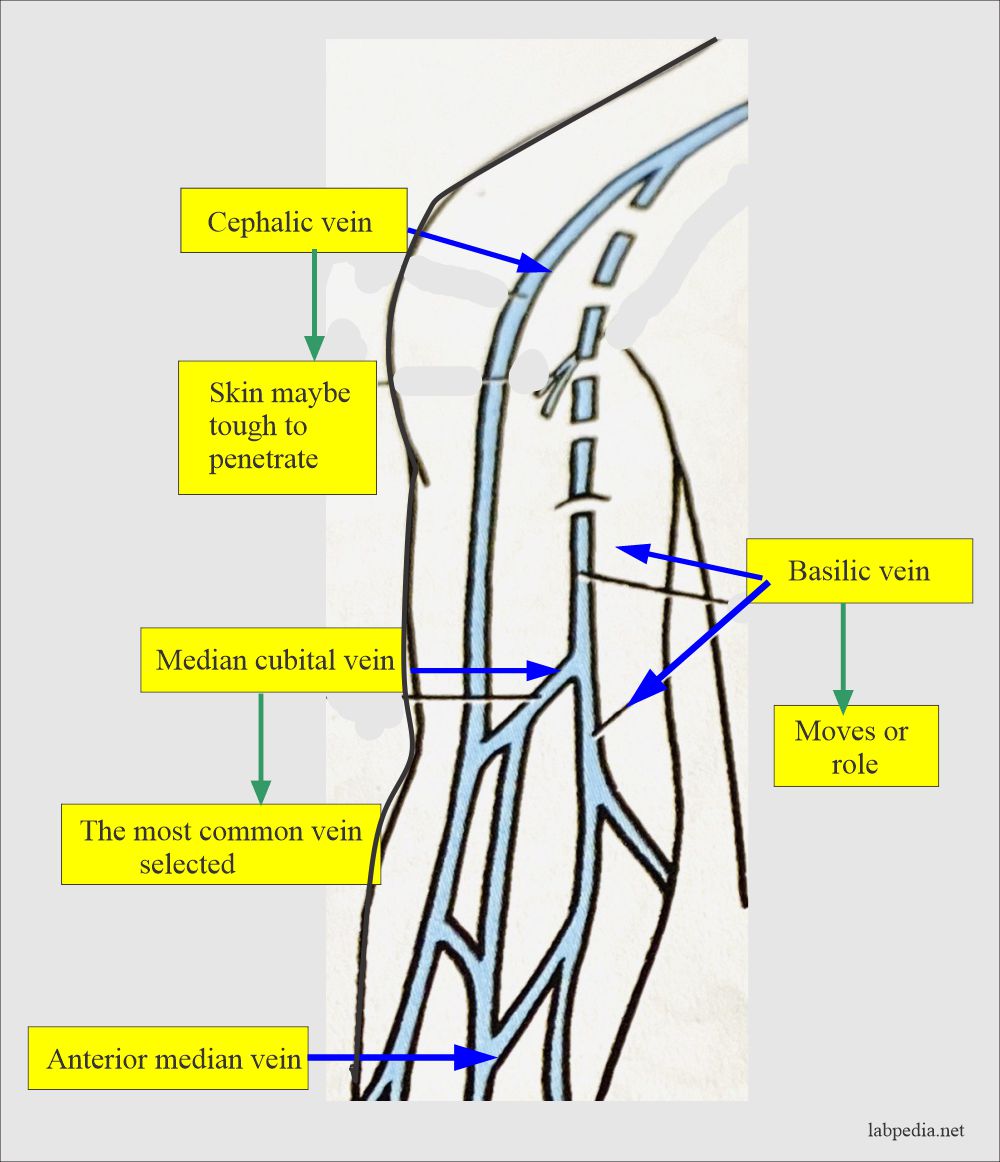
- The blood sample is taken from the forearm, wrist, or ankle veins.
- A forearm site is preferred. Blood is taken directly from the vein, called phlebotomy.
- The median cubital vein is usually preferred.
- Mostly venous blood is drawn in the fasting state.
- Blood collected after the meal is called a postprandial sample.
- There are biological variables in blood collection:
- Patient lying in bed or standing up.
- After the exercise.
- Diurnal variations.
- Recent food intake.
- Recent intake of Tea/coffee (caffeine), smoking (nicotine), alcohol ingestion, and drug administration.
- Can take a blood sample in vacutainers, syringes, and with the help of butterfly needles.
- The blood samples can also be taken for blood culture.
Difference between the values of venous and capillary blood serum:
| Capillary blood values < than venous blood | No difference in capillary and venous blood values | Capillary blood values > than venous blood. |
|
|
|
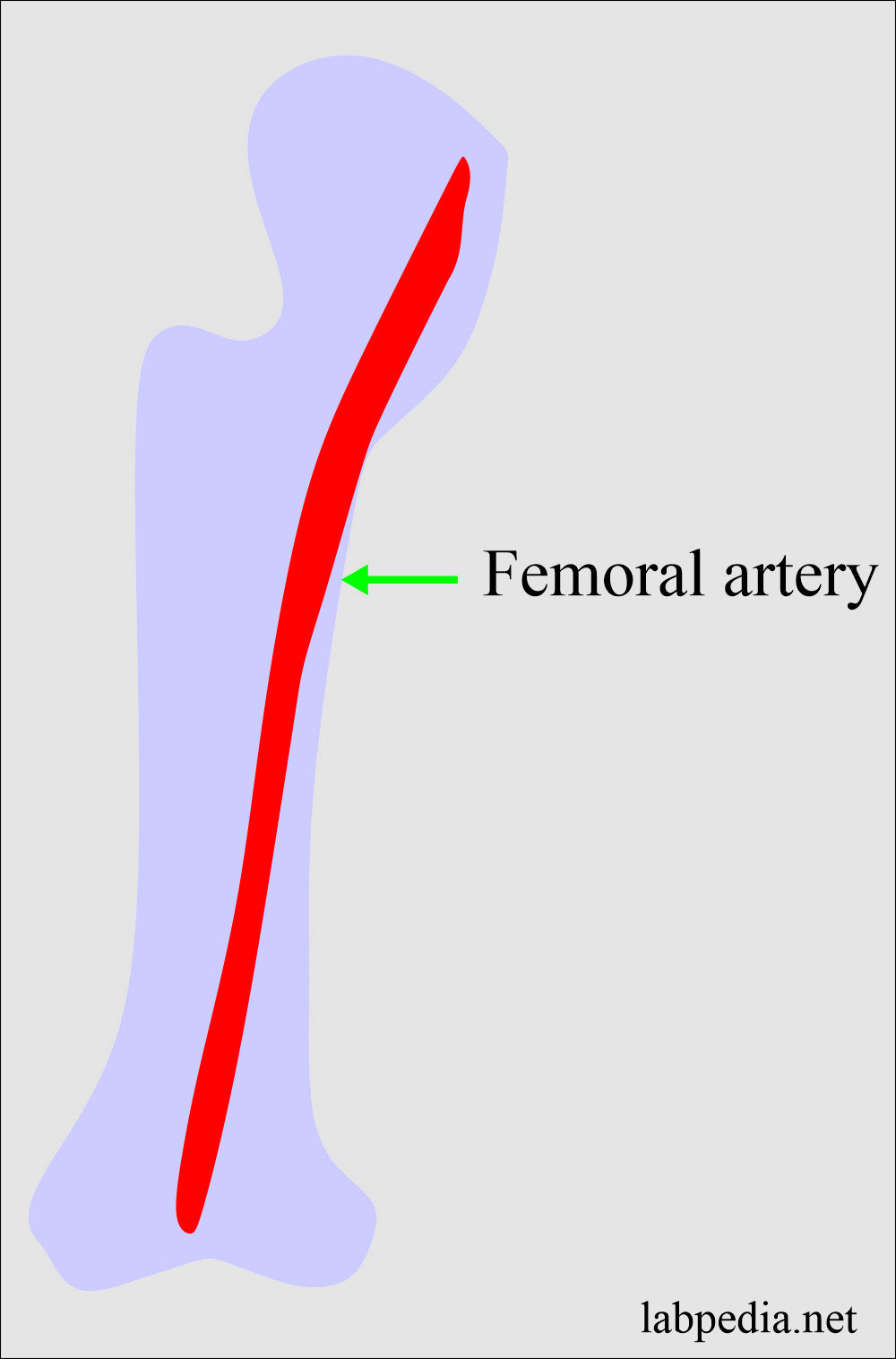
Arteria blood is needed for the blood gases:
- Arterial blood is usually taken from the femoral artery.
- Blood for gases should be processed immediately without any delay.
Types of blood samples and their indications:
| Type of blood sample | Special features | Indications |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Summary of blood samples:
Whole blood
- Obtain a blood sample in the test tube containing an anticoagulant.
- This sample will contain cells (white blood cells, platelets, RBCs, proteins) and plasma.
- This blood sample can be taken from the capillaries or the veins.
Difference between the capillary and venous blood values:
| Characteristic features | Capillary blood vs. Venous blood |
|
|
|
|
|
|
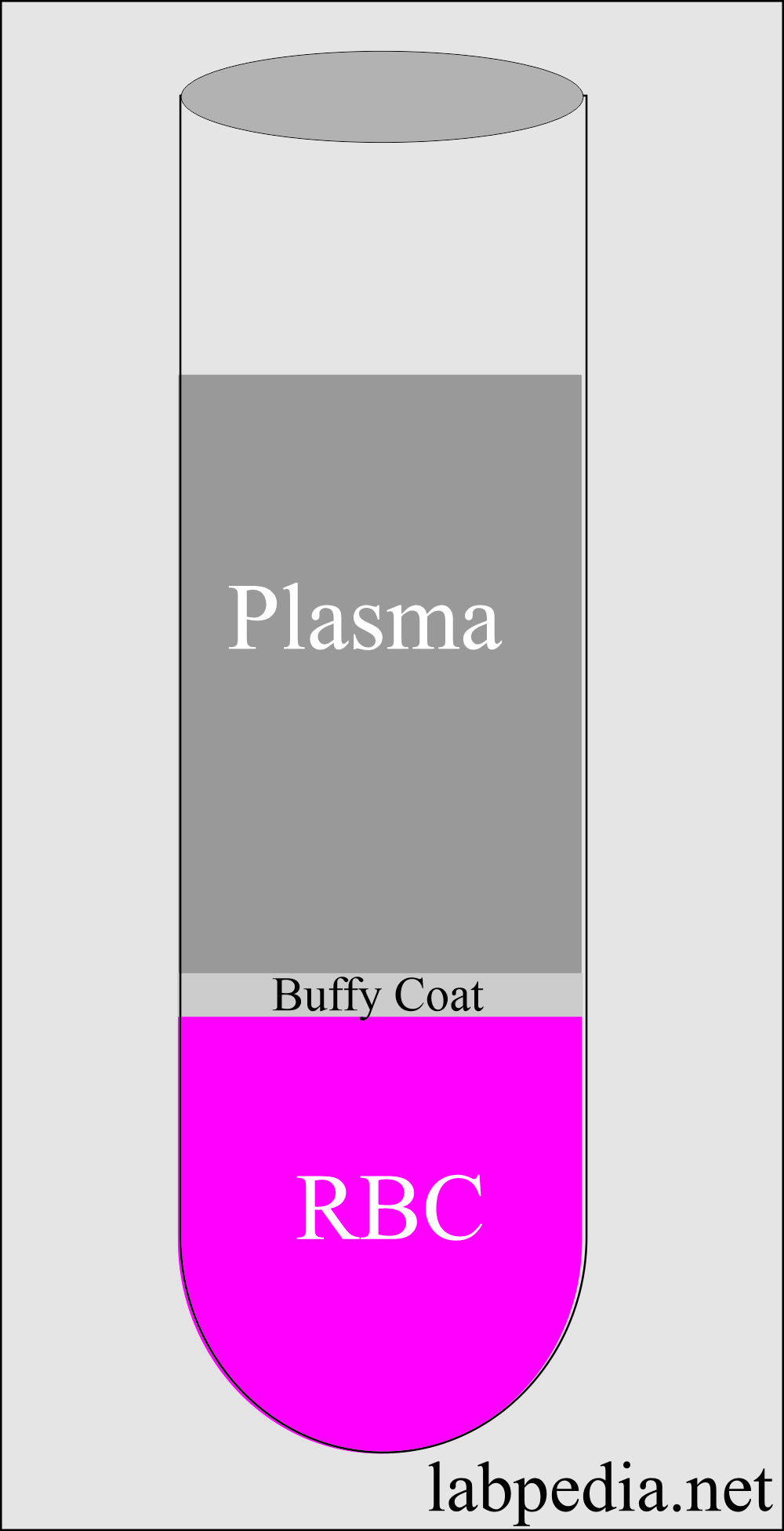
Plasma
- Plasma is 5% of the total body volume.
- This pale yellow liquid contains RBCs, white cells, and platelets.
- Plasma forms with the help of anticoagulants, which will prevent clotting.
- There is the presence of fibrinogen in the plasma.
- Plasma may have different colors due to the following:
- Total bilirubin will change color to yellow because of jaundice.
- Increased fats and lipemia change color.
- Plasma may be pink due to the presence of free hemoglobin.
- Plasma is green in color due to ceruloplasmin.
- Bacterial contamination also changed the color and appearance of the plasma.
- Excess drugs may alter the color of plasma.
- The orange-pink color is caused by carotenoids.
- Plasma color may change in various diseases.
Overall contents of the plasma:
| Substances in the plasma | Contents of the plasma |
| Water, Volume | ∼3.5 L |
| Cations | |
| Sodium (Na+) | 142 meq/L |
| Potassium (K+) | ∼4 meq/L |
| Magnesium (Mg++) | ∼2 meq/L |
| Calcium (Ca++) | ∼6 meq/L |
| Trace elements | ∼1 meq/L |
| Total cations | 155 meq/L |
| Anions | |
| Chloride (Cl–) | 103 meq/L |
| Bicarbonate (HCO3–) | 27 meq/L |
| Hydrogen phosphate (HPO4—) | ∼2 meq/L |
| Sulfate (SO4 —) | ∼1 meq/L |
| Proteins | 16 meq/L (7 g/dL = 12 to 16 meq/L of negative charge) |
| Organic acids | ∼5 meq/L |
| Total anions | 154 meq/L |
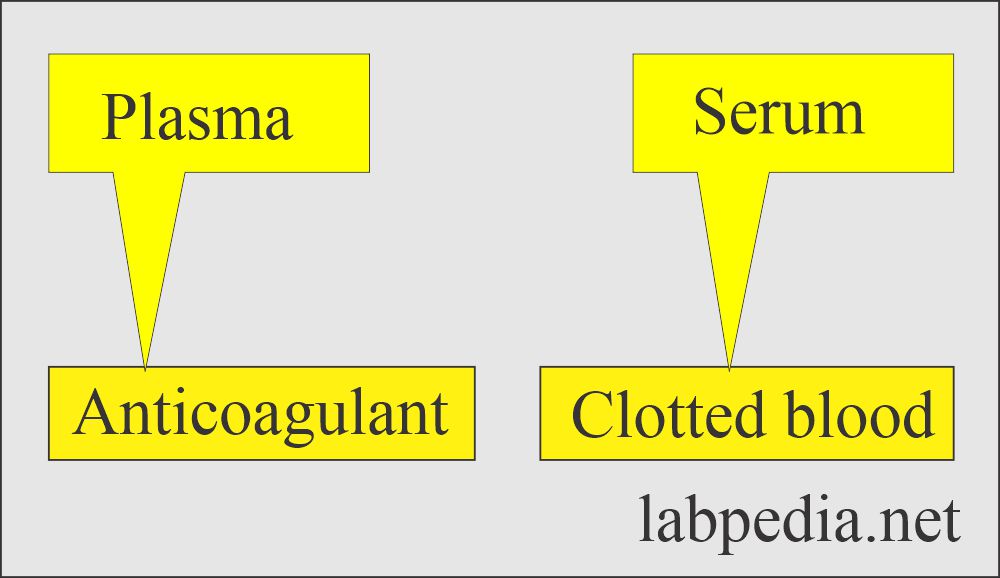
Serum
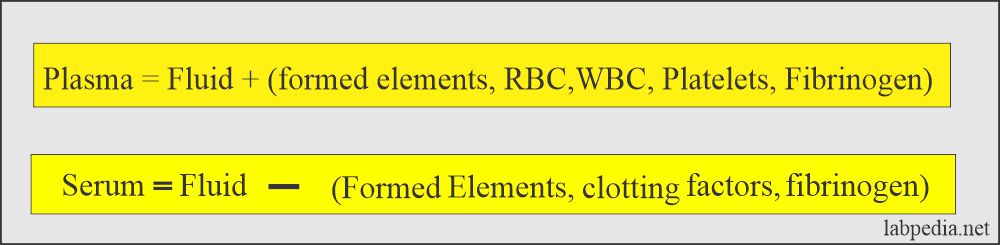
- This is a clear fluid that is separated from the clotted blood. There are no RBCs, white cells, or platelets. There is no need for anticoagulants.
- It is a liquid portion of the blood that has been allowed to clot or plasma without fibrinogen.
- Clotted blood is kept at 37 °C for at least 20 minutes and then centrifuged.
- The upper portion is called serum.
- There is no fibrinogen.
Buffy Coat
- This is the middle layer between the plasma and RBCs.
- This will contain white cells and platelets.
Table showing the difference between the contents of plasma and serum:
| Contents of plasma and serum | Plasma | Serum |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The difference between plasma and serum:
Characteristics |
Plasma |
Serum |
| Fibrinogen | 0.2 to 0.4 G/dL | Nil |
| Formation site | Present in the body fluid | Prepared outside the body |
| Outside, the body contains | Always contains anticoagulant | Never anticoagulant added |
The comparison of plasma and serum regarding the values:
| Chemical substances | Plasma values >than serum | Plasma values are< than serum. | No difference in the value of serum and plasma |
| Calcium | 0.9% | ||
| Chloride | 0.2% | ||
| Total protein | 4% | ||
| LDH | 2.7% | ||
| Albumin | 1.3% | ||
| SGOT | 0.9% | ||
| Alkaline phosphatase | 1.6% | ||
| glucose | 5.1% | ||
| Bicarbonate | 1.8% | ||
| Sodium | 0.1% | ||
| Phosphate | 7% | ||
| Potassium | 8.4% | ||
| Urea | 0.6% | ||
| Uric acid | 0.2% | ||
| Bilirubin | |||
| Creatinine | |||
| Cholesterol |
Summary of the blood samples:
| Type of blood sample | Description | Where to use |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purpose of anticoagulants:
- To prepare the whole blood or the plasma, anticoagulants are needed.
- The anticoagulants are added to the container before collecting the blood sample.
- These are used to prepare whole blood or plasma during the collection of blood samples.
Specimens to be rejected are:
- Sample with lipemia.
- Sample showing hemolysis.
- Specimens with contamination, like not proper cleaning of the site.
- The sample quantity is not enough for the proper ratio for the tests.
Anticoagulants used are:
EDTA (Ethylenediaminetetraacetic acid)
- Indications:
- This is useful for the hematological examination.
- It is used for cell count, hematocrit, hemoglobin estimation, and differential cell count.
- EDTA is used as a disodium or dipotassium salt.
- Mostly potassium EDTA is used as an anticoagulant, recommended for hematology studies. This is more soluble.
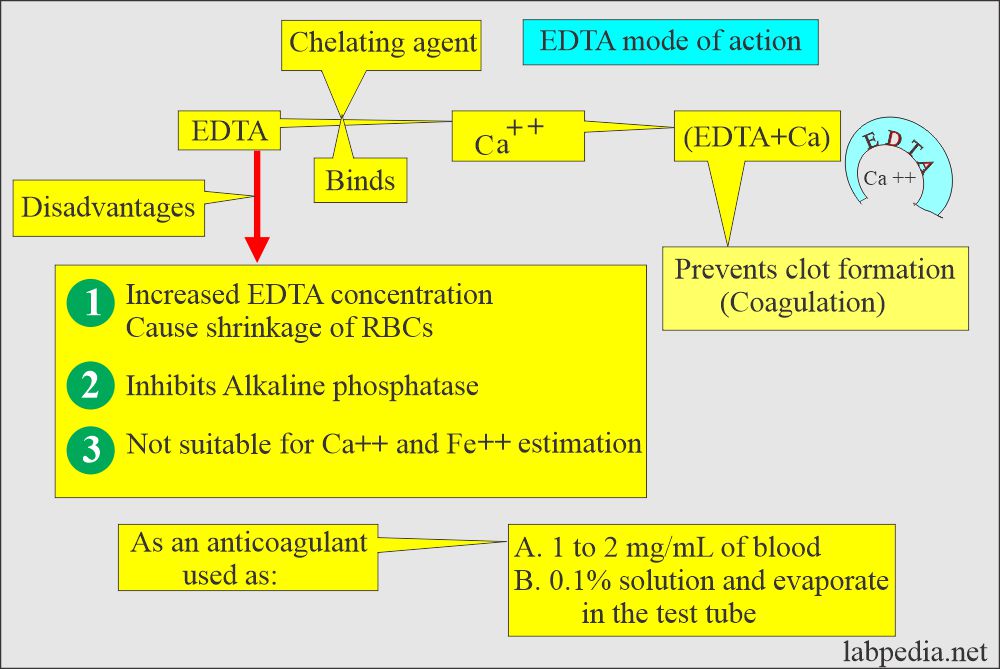
- Mechanism of action:
- EDTA is a chelating agent that binds the calcium needed for coagulation. Chelation prevents coagulation.
-
- It is effective at a final blood concentration of 1 to 2 mg /mL.
- This can be used as a powder or solution and added to vials. Let it dry.
- It is used as disodium, dipotassium, or tripotassium salt.
- Solution of EDTA:
- EDTA solution of 0.1% can be prepared and used. Let it evaporate at room temperature.
- Or 1.5 mg/mL.
- More than 2 mg/mL causes shrinkage of the cells.
- Advantages of EDTA:
- EDTA preserves the morphology of the blood cell structure.
- This is the anticoagulant of choice for hematocrit, Hb, and differential count.
- This is the best anticoagulant for peripheral blood smears and studies.
- It has little effect on the various tests.
- They produce less shrinkage of RBCs.
- There is less increase in the cell volume after keeping the blood.
- Drawbacks of EDTA:
- It inhibits alkaline phosphatase, creatine kinase, and leucine aminopeptidase activities.
- EDTA is not suitable for Calcium and iron estimation.
Heparin
Indications:
- This is used in DVT (deep vein thrombosis).
- It is used in pulmonary embolism.
- This is also used in unstable angina.
- This is used as a prophylactic drug in venous thrombosis.
- If needed in pregnancy, this is the drug of choice because it can not cross the placenta.
- This is used in cardiopulmonary bypass surgery. This will maintain the patency of the blood vessels.
- It can be used in DIC if there are predominantly vasoocclusive manifestations.
- Low molecular weight heparin is given subcutaneously because this has a longer half-life than heparin.
- A prophylactically single dose is needed. Lastly, it is used as an anticoagulant and is mostly used in hematology.
Properties of Heparin:
- This is an anticoagulant and causes the least interference with the test.
- This is theoretically the best anticoagulant because it is a normal blood component and does not introduce any foreign contaminants to the blood specimen.
- This acidic mucopolysaccharide with a molecular weight of 15,000 to 18,000 is a blood coagulation inhibitor by potentiating the antithrombin activity.
- This is more costly than the others.
- It is present in powder form but is hygroscopic and dissolves rapidly.
- Mucoitin poly sulfuric acid is available as sodium, potassium, lithium, and ammonium salts.
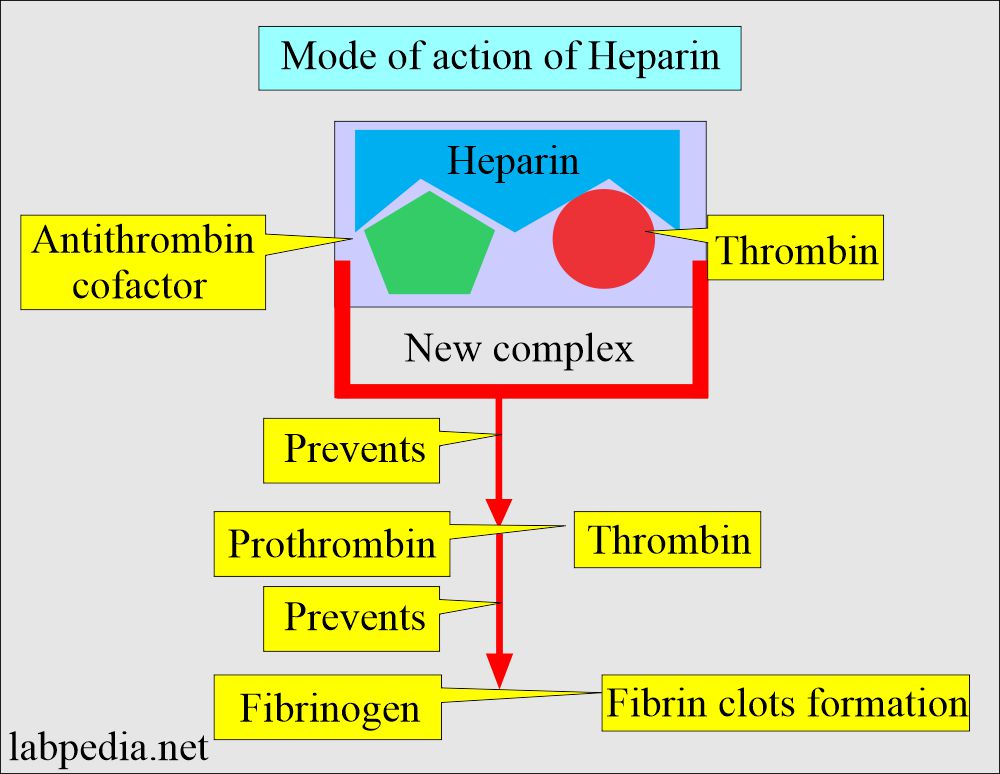
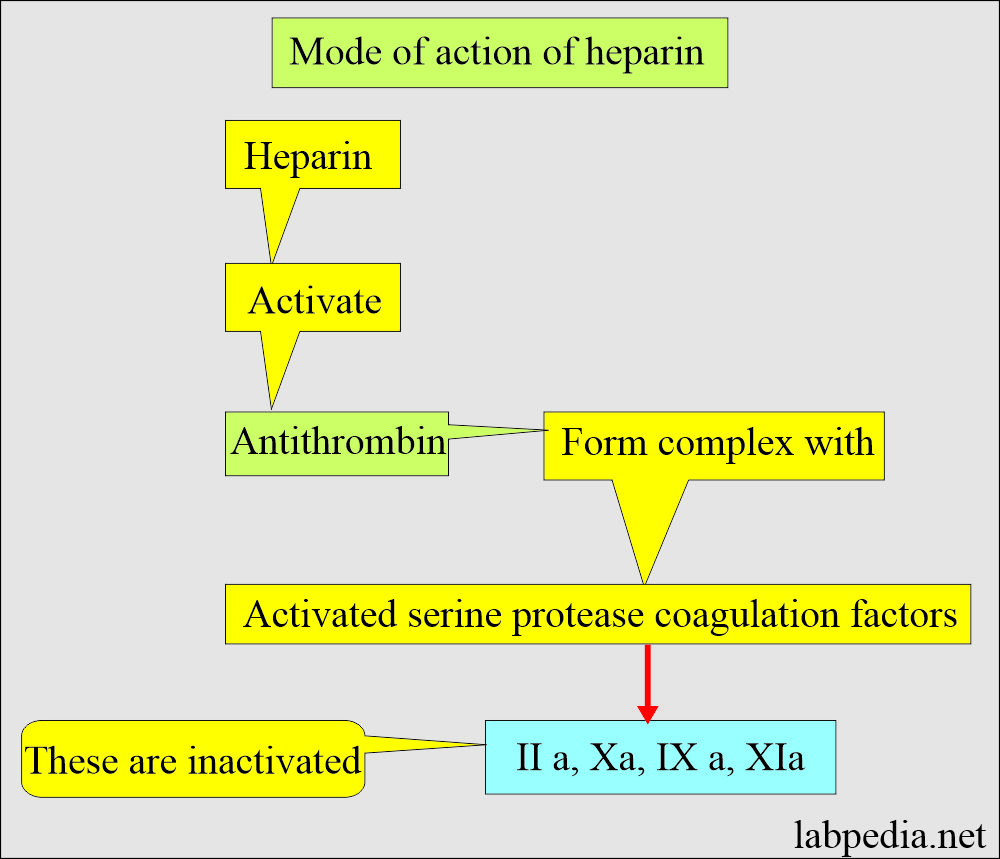
- Mechanism of action of heparin:
- The GI tract does not absorb it, so given by injection in case of therapy.
- Heparin accelerates antithrombin III action, neutralizing thrombin, thus preventing fibrin formation from fibrinogen.
- It forms the thrombin + antithrombin cofactor + heparin complex, preventing fibrin clot formation.
- It prevents coagulation for 24 hours by neutralizing the thrombin, thus preventing fibrin clots’ formation from the fibrinogen.
- Solution preparation of the heparin:
- Each test tube adds Heparin 0.2 mg /mL of blood.
- Or 20 units of heparin for 1 mL of blood (in another reference, 15 U/mL).
- Or a drop of heparin is drawn into the syringe.
- Or simply coating the inside of the tubes or syringe is enough for the anticoagulant effect.
- After collecting blood, invert the tubes 5 to 7 times for proper blood mixing.
- Each test tube adds Heparin 0.2 mg /mL of blood.
- Advantage:
- This is the best anticoagulant to dry when minimal hemolysis is desired, e.g., sodium and potassium estimation.
- This is the best anticoagulant to estimate pH, blood gases, electrolytes, and ionized calcium.
- Drawback
- It is costly.
- It inhibits the acid phosphatase activity.
- It gives a blue background for Wright’s stain smears, so not good for peripheral blood smear interpretation.
- It also affects the binding of triiodothyronine and thyroxine to their carrier protein and produces a higher free concentration of these hormones.
- It interferes with the binding of calcium to EDTA.
- It is not used for coagulation and hematology studies.
- Ammonium heparin affects the RBC volume.
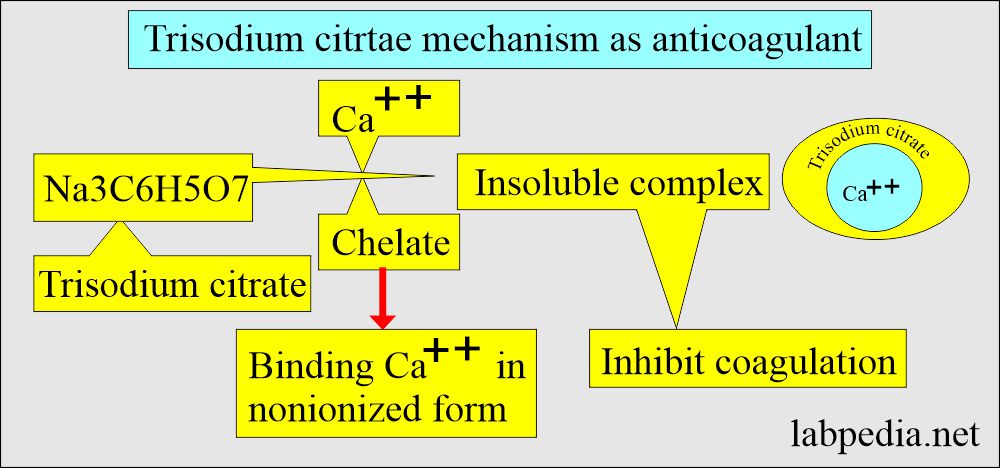
Sodium Citrate
- Citrate is used as trisodium citrate salt.
- It is a white hygroscopic crystalline powder.
- Indications:
- Sodium citrate is widely used for coagulation studies.
- For PT and PTT.
- The sample can be used for ESR by the Westergren method.
- Mechanism of action:
- It is used in solution form.
- This will chelate calcium. Inactivates Ca++ ions.
- This will prevent the rapid deterioration of labile coagulation factors like factor V and factor VII.
- Sodium citrate solution preparation and uses:
- Trisodium citrate= 3.2 to 3.8 g/dL (3.2% solution).
- Mix well Trisodium citrate 3.8 grams in distle water.
- This can be used as 0.109 mg/mL.
- In blood, its ratio is 1:9, where 9 parts are blood, and 1 part is sodium citrate.
- PT and PTT= Blood: Sodium citrate = 9: 1 part (blood 9 parts: sodium citrate 1 part)
- ESR = Blood: Sodium citrate = 4:1 (1.6 mL of blood: o.4 mL Sodium citrate).
- Drawbacks of sodium citrate
- This is used in liquid form (liquid anticoagulant).
- This is not a good anticoagulant for a complete blood examination.
- This is not good for the estimation of calcium.
- It inhibits aminotransferase and alkaline phosphatase.
- This will stimulate acid phosphatase when phenyl phosphate is used as the substrate.
- It has little value in clinical chemistry.
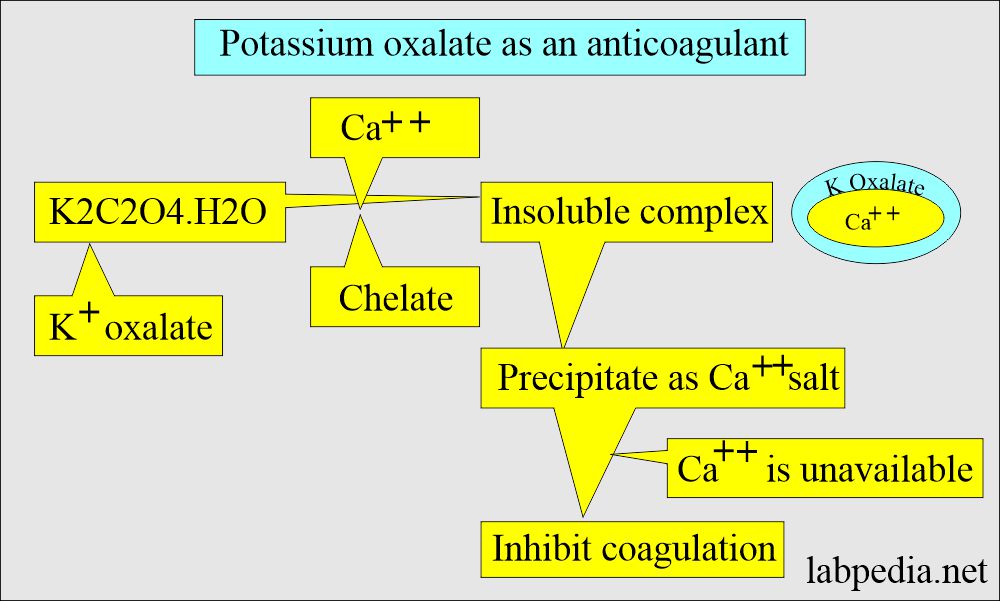
Potassium Oxalate
- Mechanism:
- This may be sodium, potassium, ammonium, or lithium oxalic acid salt used as an anticoagulant.
- This forms an insoluble complex with calcium ions (precipitate with calcium as a salt).
- This is the most popular oxalate salt used as an anticoagulant in powder form.
- How to prepare K-oxalate Solution:
- Potassium oxalate is used at a concentration of 1 to 2 mg/mL of blood.
- Bulk solution: when you mix 30 grams/dL in distal water.
- Now add a few drops to the test tube side and dry it in the oven below 100 °C.
- The combination of ammonium/potassium oxalate does not lead to shrinkage of the RBCs.
- While other oxalates cause shrinkage.
- Drawbacks of potassium oxalate
- If the concentration is >3 mg/mL, there are chances for hemolysis.
- There is a reduction of 10% in hematocrit.
- Oxalates inhibit enzymes like acid phosphatase, alkaline phosphatase, amylase, and LDH.
- It may cause the precipitation of calcium as oxalate salt.
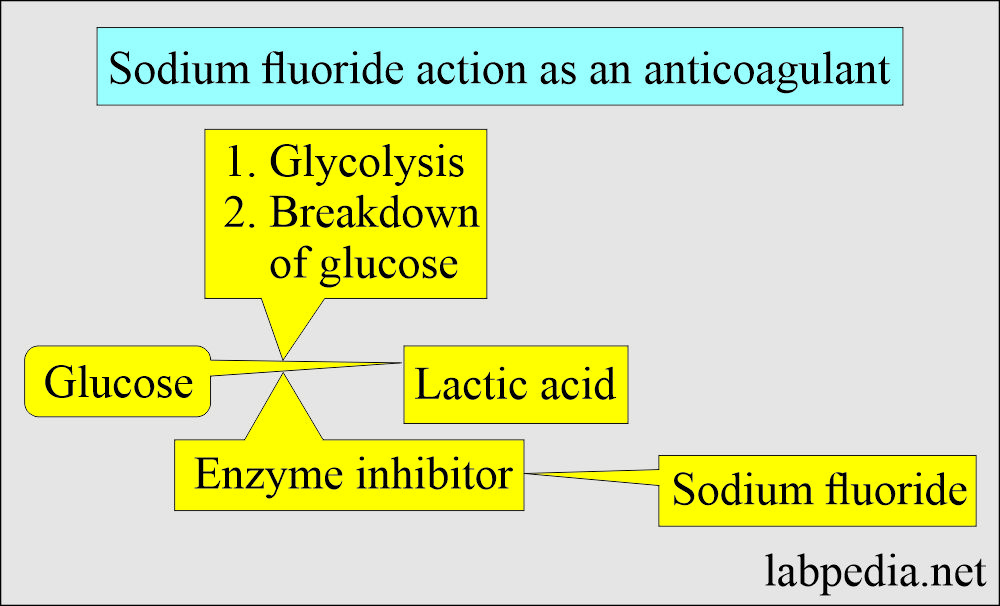
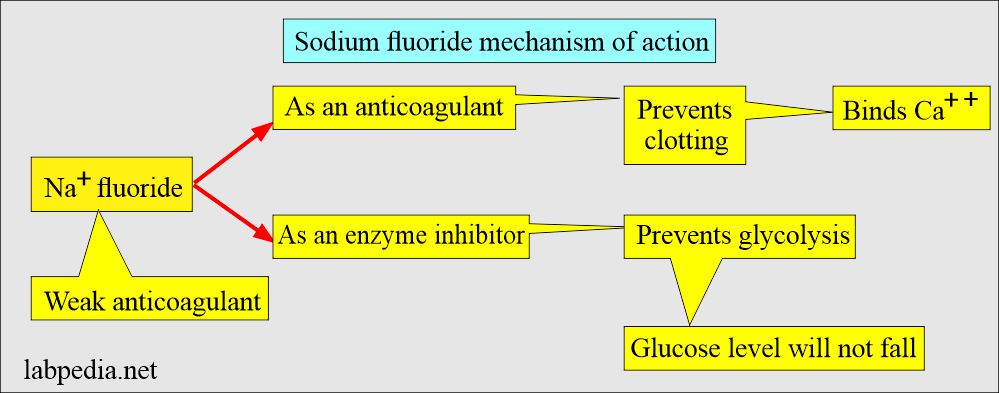
Sodium Fluoride
- This is a weak anticoagulant but uses an antiglycolytic agent to preserve glucose.
- This inhibits the system involved in glycolysis and preserves the glucose.
- This can be used as a dry additive.
- Mechanism of action: It acts in two ways:
- As an anticoagulant, by binding the calcium.
- As an enzyme inhibitor that prevents the glycolytic enzyme from destroying the glucose.
- Sodium fluoride acts after the enolase, so it will not be effective in the first 1 to 2 hours. It prevents glycolysis after this period.
- Glucose can fall during this period, around 10 mg/dL.
- Transporting on ice and rapid serum separation within 30 minutes can prevent glycolysis. There is no need for the addition of sodium fluoride.
- Not good for clinical chemistry tests.
How to prepare sodium fluoride Solution:
- This is effective at 2 mg/mL of blood concentration and another anticoagulant like potassium oxalate.
- When used alone, more concentration than 2 mg/mL is needed.
- This can be used in combination with oxalate as a fluoride-oxalate mixture.
- Most specimens are preserved at 25 °C for 24 hours and at 4 °C for 48 hours.
- Sodium fluoride is poorly soluble, so mix blood thoroughly before effective anti-glycolysis occurs.
- This is mainly used for glucose estimation.
- The rate of decrease is faster in newborns because of the increased metabolic activity of the white cells.
- Drawback
- This is also an inhibitor of many enzymes.
- Also, effect urease for the estimation of urea.
Sodium Iodoacetate
- This is an effective antiglycolytic agent and substitute for sodium fluoride.
- This does not affect urease for glucose and blood urea levels; instead, sodium fluoride or a single sample.
- Solution use:
- It can be used at a concentration of 2 g/L and is an effective glycolytic agent.
- This may be substituted for sodium fluoride.
- This does not affect urease.
- Drawback:
- It inhibits creatine kinase but does not affect other chemistry tests.
The anticoagulants and types of blood samples:
| Type of the blood sample | Anticoagulant added | Mechanism of action | Use of the blood sample |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Adverse effects of the additives:
- The additive may contain the substance to be tested, like Na+oxalate, to estimate Na+.
- The additive may remove the component tested, like in oxalate, removing the calcium.
- The additive may affect enzymes like Na+flouride. This may destroy many enzymes.
- A small amount of the anticoagulant gives rise to microclots, interfering with cell count.
- The additive may distort the cells like oxalate and change cell morphology like RBCs, which will become crenated. While WBCs show vacuoles. Lymphocytes and monocytes will have distorted shapes.
- If the excess quantity is used, that will dilute the substance to be tested.
Questions and answers:
Question 1: Why is sodium fluoride a good anticoagulant for estimating glucose?
Question 2: What is the difference between plasma and serum?


Good
Thanks
Good explanation on the anticoagulations of analytes in the Clinical Chemistry lab
Thanks
I agree with you
Thanks.
It’s hard to say
Sir please provide pdf
Please you can do a PDF file yourself.
Really appreciate this sir, can you make it to PDF so that we can download it.. Thank you
Thanks for the appreciation. We are considering but don’t have many options at this time.
Thank you for this explanation. I am having trouble finding a good reference regarding depressed glucose results due to improper mixing in grey tops. The statement “Sodium fluoride is poorly soluble, so mix blood thoroughly before effective anti-glycolysis occurs” makes complete sense to explain why improper mixing could yield lower results. Do you have any further reference for this effect?
Sodium fluoride, according to one reference weak anticoagulant, so you can use it with another anticoagulant. Its main function is preservative by inhibiting the enzyme system involved in glycolysis. Its concentration is 2 mg/mL when mixed with another anticoagulant, but you need 3 to 5 times greater concentration when used alone than the usual 2 mg/mL. This higher concentration and inhibition of the glycolytic cycle is likely to cause a fluid shift. This can not be justified to use for other chemistry tests.
Thank you so much for this outline. It’s a wonderful explanation of anticoagulants & their mechanisms of action. I’ve saved it for my own references purposes. Perfect for new techs & phlebs or as a refresh for anyone who might need it.
Thanks.
Thanks you sir
Welcome.
Sir I am writing a book can I use some images of your labpedia.net
in my book because sir in a image you explain very well so sir pleas
Please see your email.
Thank you So much sir. You are very soft heart person.
well summarized notes, thank you
Thanks.
Hi sir,
I am writing a report on testing chloride in the body but I’m not sure of the method, sample types, the reagents or the preservatives involved.
Please see this link:
https://labpedia.net/chloride-blood-chloride-cl%c2%af-and-cystic-fibrosis/
Thanks for this good information .Sir i want to know the term what is true glucose?
True glucose is estimated by the enzymatic method. At the same time, Benedict’s reaction gives other reducing substances.
True glucose is measured by the enzymatic method. In comparison, Benedict’s solution estimates all reducing substances.
Hello doc Riaz. May I ask how can I preserve a serum?
You can keep serum for short period in the fridge. For a longer period freeze the serum. If you want to keep it for more than 6 months freeze it and keep it at -20C (or -70C).
Sir, I must really commend your work. I would love to make more research on this but I don’t seem to find the references you used for this work
This topic is covered in various books and my personal experience in my practice.
hello sir
Please let me know if I can be of any help.
hello sir ,i am manufacturer of blood collection tube, I need solution making formula for anticoagulants like EDTA,SODIYAM FLORIDE and CLOT activator with concentration for best result test.please help
https://labpedia.net/blood-sample-types-anticoagulants-preservatives-adverse-effect-of-additives/
Please read this article; most solution preparations are given in this article.
This is a good, educative and impressive website for medical laboratory student. It’s very easy to read and understand.
Thanks.
Thank you for this thread. It is very helpful for a pathology resident. I wish you had “advantages” for all the anticoagulants. This would make it easier to compare all of them.
Please read carefully; I have discussed the advantages and disadvantages of the anticoagulants.
Thank you sir for your valuable inputs.. Its really helpful.. Sir can you please guide us on clot activator reagents.. how can we prepare clot activator solution ??
I think there are ready made tubes with clot activator.
There are:
1. Silica-based clot activator.
2. Thrombin clot activators.
I will suggest ready-made test tubes.
¡Excelente trabajo! Soy Estudiante de Bacteriología y debo reconocer el fundamento de cada tubo. ¡Saludos desde Colombia, Dr. Riaz!
Thanks.
Whether sodium sodium fluoride with oxalate tubes can be used for haematology tests also?
Please see these comments about oxalate for hematology.
Oxalate is not good for hematology studies.
Drawbacks of potassium oxalate
If the concentration is >3 mg/mL, there are chances for hemolysis.
There is a reduction of 10% in hematocrit.
Oxalates inhibit enzymes like acid phosphatase, alkaline phosphatase, amylase, and LDH.
It may cause the precipitation of calcium as oxalate salt.
Hello sir,about peripheral blood and venous blood, do we need to use different proportions of heparin lithium anticoagulant tubes for biochemical testing? I see that the market has described the use of peripheral blood and venous blood, and their tube anticoagulant ratio is not the same
Peripheral blood smear is done on the venous blood. For biochemical testing the best sample is serum. Plasma is not preferred sample.
Sorry,I should describe Capillary blood and venous blood.
When you take capillary blood then you will make direct smear. No need to put into anticoagulant. Capillary blood is mostly used directly.
Please see this topic:
https://labpedia.net/blood-sample-types-anticoagulants-preservatives-adverse-effect-of-additives/