Glucose-6-Phosphate Dehydrogenase Deficiency (G6PD)
What sample is needed for Glucose-6-Phosphate Dehydrogenase Deficiency Anemia?
- Collect the venous blood in lavender top or green top test tubes.
What are the indications for Glucose-6-Phosphate Dehydrogenase Deficiency?
- This test is used to find Glucose-6-Phosphate Dehydrogenase Deficiency Anemia.
- It is useful in males susceptible to these genetic defects.
What is the history of the Glucose-6-Phosphate Dehydrogenase Deficiency(G6PD)?
- G6PD deficiency was directly identified in the 1950s from investigations of the hemolytic anemia effect of the antimalarial drug primaquine.
- Hemolytic anemia due to antimalarial drugs was reported in Pnanamian plantation workers.
- During the Korean War, this hemolytic anemia due to antimalarial drugs was described by labeling the RBCs.
- Carson and associates identified the enzyme G6PD deficiency in 1956 in an individual who developed hemolytic anemia after taking the antimalarial drug primaquine.
- Yoshida first isolated the purified enzyme from human RBCs in 1966.
- Later, the variants of the G6PD were identified by sequencing amino acids, DNA cloning, and nucleotide sequencing.
- Recent advances in molecular biology have enabled the classification of the variants of G6PD deficiency, and there are around 50 gene mutation groups.
- Another reference says >400 variants are present.
What is the incidence of Glucose-6-Phosphate Dehydrogenase Deficiency?
- There is a high prevalence in Africa of G6PD deficiency.
- There has also been an increased incidence in Southern Europe, the Middle East, Southeast Asia, and Oceania.
- G6PD deficiency affects 12% of African Americans.
How will you define Glucose-6-Phosphate Dehydrogenase Deficiency?
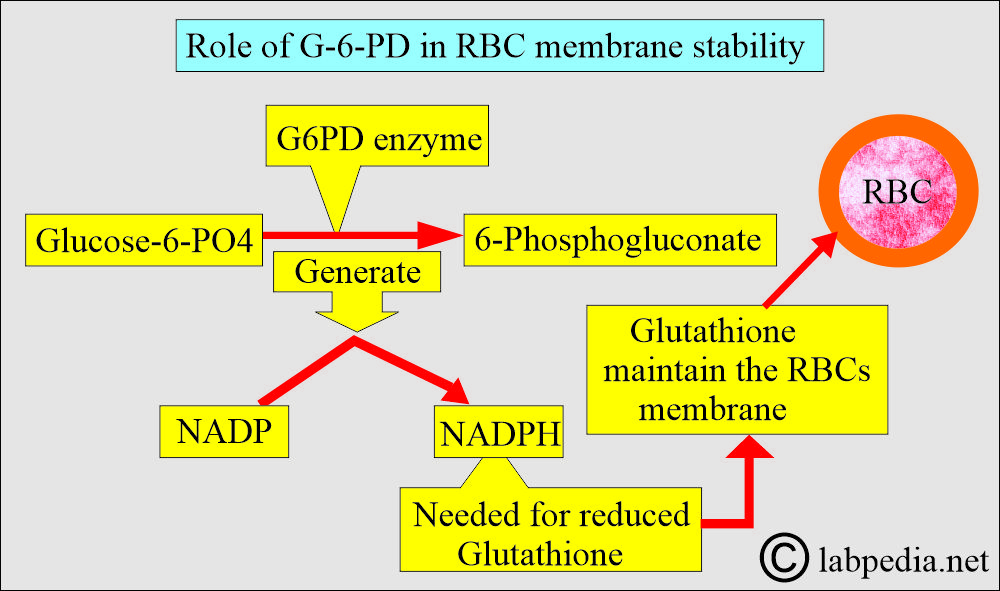
- G6PD is the enzyme that catalyzes the first step in the hexose monophosphate pathway, converting glucose-6-phosphate to 6-phosphogluconate and generating NADPH from NADP. NADPH produces a reduced form of glutathione, which helps maintain the RBC membrane.
What is the pathophysiology of G6PD deficiency?
- This is an X-linked (sex-linked) genetic abnormality of glucose-6-phosphate dehydrogenase deficiency, which is an inborn error of the RBCs.
- The female X chromosomes carry this.
- The defect is more severe in males (XY) and a much smaller number of females in whom both XX chromosomes have abnormal genes.
- If only one X chromosome is abnormal in females, they are carriers. These may be asymptomatic to moderately abnormal, even after stimulation.
- The gene for G6PD is localized to chromosome Xq28 near the factor VIII gene.
- The main clinical S/S is seen in males.
- This will protect against the Plasmodium spp infestation.
- Reticulocytes have more normal G6PD activity than the older RBCs.
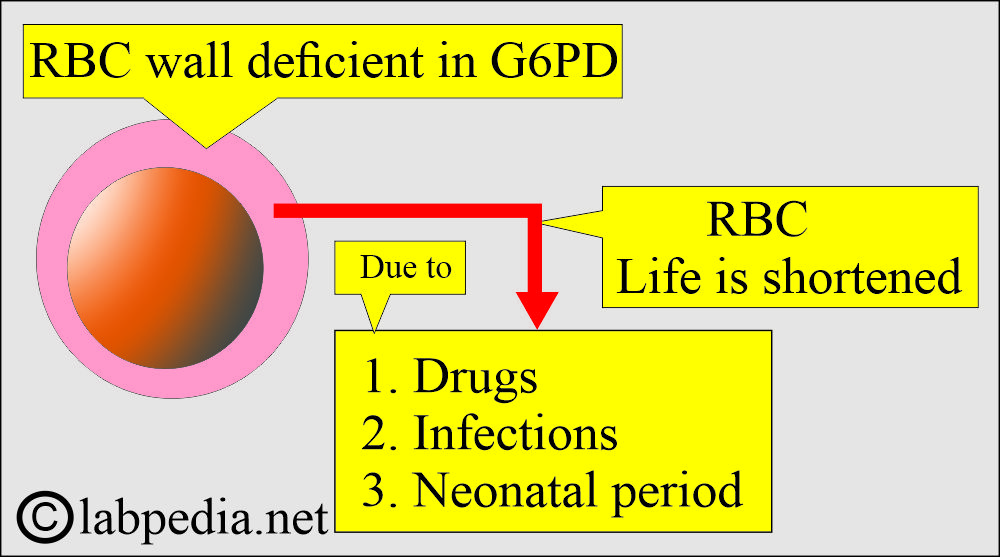
- Deficient RBCs of G6PD are more fragile and short-lived.
- This disease is prevalent in Africa, South Europe, the Middle East (around 20%), South East Asia (around 49% in some regions), and Oceania.
- Overall,> 400 million people are affected by enzyme deficiency.
What is the basic mechanism of Glucose-6-Phosphate Dehydrogenase Deficiency?
- There are two types of disease-causing mutations:
- Mediterranean = G6PD med.
- This mutation is more severe because every new RBC is deficient in the G6PD enzyme.
- The G6PD enzyme deficiency is 10% of the normal.
- African = G6PDA.
- Young RBCs (reticulocytes) maintain an adequate G6PD enzyme level for a longer time.
- G6PD activity is around 20% to 60%.
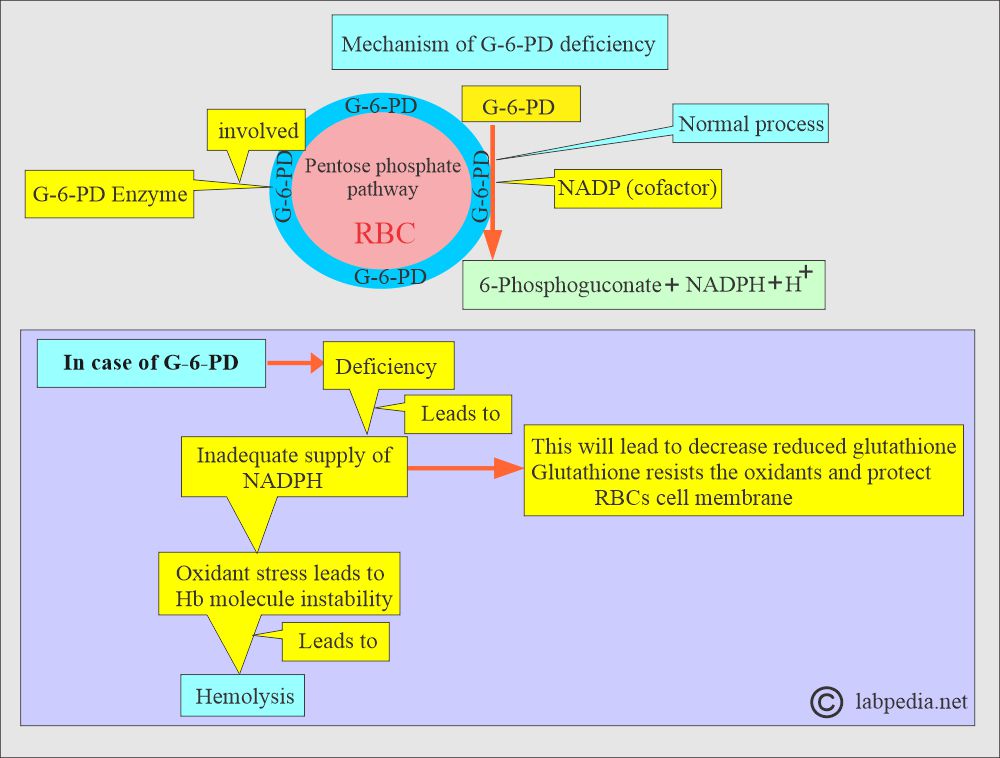
- G-6-PD is the initial enzyme involved in the pentose-phosphate pathway of RBC metabolism.
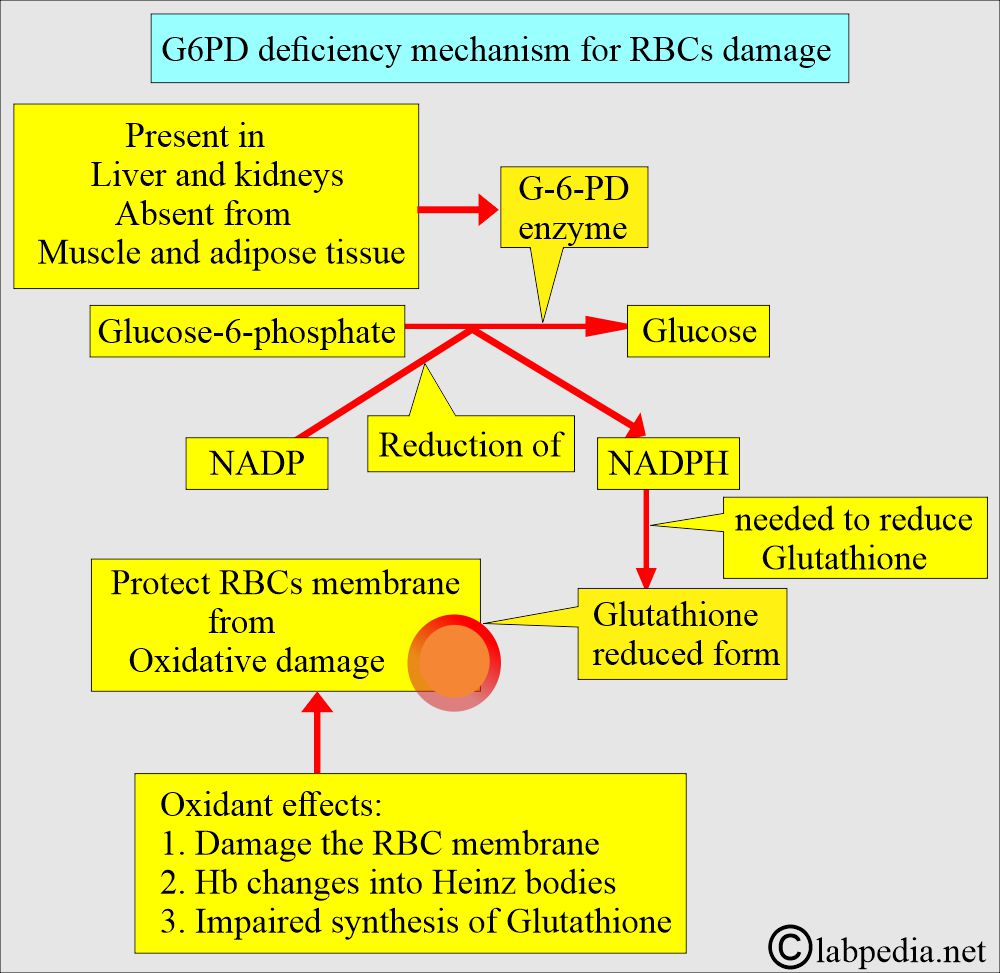
- G6PD is involved in producing NADPH, which maintains glutathione and other proteins in the reduced state and protects them from oxidants.
- When hemoglobin is oxidized, it will precipitate as Heinz bodies.
- When passing from the sinusoids of the spleen, the macrophagic cells target the Heinz bodies containing RBCs.
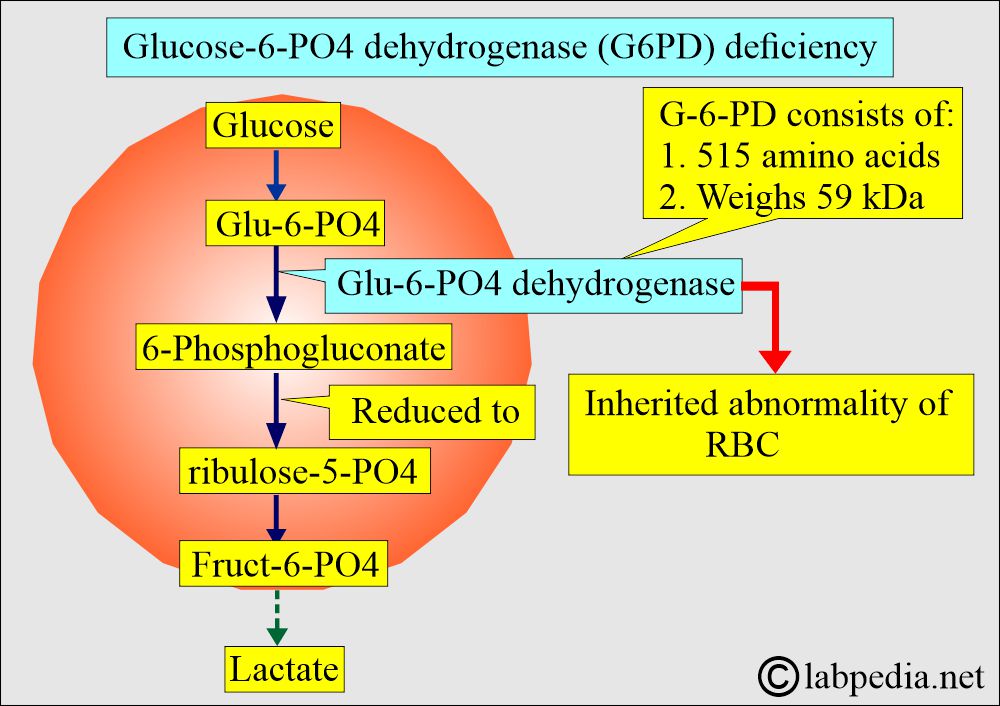
- It catalyzes the removal of H+ ions from glucose-6-phosphate to produce 6-phosphogluconate and requires a cofactor, NADP.
- NADP is reduced to NADPH, preventing the effect of oxidants on RBCs.
- G-6-PD produces NADPH, which maintains the Glutathione and other proteins when the RBCs are exposed to oxidants.
- In G-6-PD deficiency, there is a shortage of NADPH, and RBCs can not neutralize the oxidant stress and are hemolyzed.
- Exposure to oxidant stress damages the RBCs’ membrane and gives rise to Heinz bodies (denatured hemoglobin) formation.
- RBCs containing the Heinz bodies will have difficulty crossing the splenic pulp and will not be rapidly eliminated from circulation.
- A gene on the X-chromosome determines the structure of G-6-PD, which gives remarkable polymorphism in the human population.
- Reticulocytes are normal at the G6PD enzyme level compared to the older RBCs.
What is the structure of the Glucose-6-PO4 (G6PD)?
- It is a dimer form, which is quite common.
- Or tetramer form (these are pH-dependent).
- It consists of identical subunits.
- There are 515 amino acids.
- It weighs 59 kDa.
How will you classify G6PD deficiency based on the amount of the enzyme?
- G6PD may be associated with different clinical syndromes.
- Class 1, where there is <5% of normal RBCs enzyme activity.
- This is a rare disease.
- It is chronic, congenital, and nonspherocytic hemolytic anemia.
- This condition is worsened by oxidant drugs or febrile illness.
- This condition does not improve with splenectomy.
- Class 2, where there is <10% of the normal RBCs enzyme activity.
- The oxidant drugs like Primaquin, sulfonamides, and acetanilid may induce acute hemolytic crises.
- This condition may be seen in acidosis.
- There is no improvement by splenectomy.
- Class 3 is 10% to 60% of the RBCs’ enzyme activity.
- These patients may have hemolytic crises induced by the oxidant drugs or infections.
- There is hemolysis, which is self-limiting and subsides in 2 to 3 days.
- This is also seen in hepatic coma, hyperthyroidism, myocardial infarction, megaloblastic anemia, and chronic blood loss.
- Class 4 shows very mild or no deficiency of the G6PD enzyme.
- Class 5 shows increased activity (only one such variant was described).
- Classes 2 and 3 represent 90% of the cases. Classes 4 and 5 show no clinical signs or symptoms.
What are the stimuli for Glucose-6-Phosphate Dehydrogenase enzyme?
- Vegetables like Fava beans.
- Drugs:
- Antimalarial drugs like:
- Primaquine.
- Pamaquine.
- Chloroquine.
- Fansidar.
- Maloprim.
- Quinine.
- Antibacterial drugs like:
- Sulphonamide.
- Sulphones like co-cotrimoxazole, sulfanilamide, dapsone, and salazopyrin.
- Nitrofurans.
- Chloramphenicol.
- Ciprofloxacin.
- Dapsone.
- Analgesics like:
- Asprin.
- Acetanilide.
- Phenacetin
- Antihelminths like stibophen and β-naphthol.
- Other agents are vitamin K, probenecid, naphthalene, nalidixic acid, dimercaprol, and phenylhydrazine.
What are ethenic groups showing Glucose-6-Phosphate Dehydrogenase Deficiency (G6PD)?
- African American females, 3% and 20%, are the carriers.
- African American males are 13%.
- It is also seen in other ethnic groups like Greeks, Sephardic Jews, and Sardinians.
- It is seen in all patients with sensitivity to fava beans (favism).
What are the causes of increased Glucose-6-Phosphate Dehydrogenase (G6PD) ?
- In the case of ITP, it becomes normal after the splenectomy.
- Pernicious anemia is 3 times the normal level and remains elevated for several months, even after treatment with vitamin B12.
What will be the clinical presentation of Glucose-6-Phosphate Dehydrogenase Deficiency?
- Usually, these patients are asymptomatic and are more common in males than females.
- G6PDmed mutation is more severe, and even the very young RBCs are depleted of the G6PD enzyme.
- G6PDA is more common in the USA. Young RBCs (reticulocytes) have an adequate level of G6PD enzymes.
- G6PD activity is in the range of 20% to 60%.
- Hemolytic anemia starts:
- Acute hemolytic anemia in response to oxidant stress, such as fava beans or infections.
- There is intravascular hemolysis,
- There is hemoglobinuria.
- Neonatal jaundice.
- Rarely, there is congenital non-spherocytic hemolytic anemia. This may be because of some other enzyme deficiency.
- G6PD can be divided into:
- Type A is present in about 20% of black Africans with reduced G6PD activity.
- Type B is present in almost all Caucasians and 70% of black Africans with normal activity.
- The main races affected are West Africa, the Mediterranean, the Middle East, and South-East Asia.
- Severe deficiency occasionally occurs in white people.
- The commonest presentation is neonatal jaundice.
- There is congenital hemolytic anemia.
- The patients may have drug-induced hemolysis.
- Mostly, hemolysis starts after eating the fava beans, which is called favism.
- Most of the patients do not have any symptoms.
- RBC number is normal, and functions and survival are normal unless exposed to oxidative stress.
- Antimalarial drugs, such as primaquine, directly lead to hemolysis.
- Summary of the G6PD deficiency:
- Drug-induced hemolysis.
- Favism: Acute hemolytic anemia in response to oxidant stresses like fava beans.
- Infection-induced hemolysis.
- Neonatal jaundice.
- Rarely is a congenital non-spherocytic hemolytic anemia.
- Drugs that may increase the G6PD level are:
- Vitamin C (ascorbic acid).
- Vitamin K.
- Aspirin and phenacetin.
- Primaquine, and quinidine.
- Sulfonamides.
- Nitrofurantoin.
- Tolbutamide.
What is the Differential diagnosis of glucose-6-PO4 deficiency?
- G6PD deficiency needs to be differentiated from:
- Drug-induced hemolytic anemia is associated with unstable hemoglobinopathies.
- Enzyme defects in the pentose-phosphate shunt, like γ-glutamyl cysteine synthetase, GSH synthetase, and possibly glutathione reductase, may mimic G6PD deficiency.
- Stability tests and electrophoresis can differentiate hemoglobinopathies. Both of these tests are normal in G6PD deficiency.
- G6PD enzyme assay or fluorescent screening test will be positive only in G6PD deficiency.
Favism:
How will you define Favism?
- Favism is the grave end result of G6PD deficiency of the RBCs.
- Hemolysis may be sudden in onset. This is reported within the first hours after exposure to the fava beans.
- In most cases, onset is gradual; hemolysis is noticed 1 to 2 hours after the beans’ ingestion.
- The urine will become red or quite dark.
- In severe cases, the patient may go into shock.
What are the Lab findings of G6PD deficiency due to Favism?
- In between the crises, the blood picture is normal.
- Hemoglobin varies from 7 g/dL to normal.
- Typically, following the drug exposure, there is an acute drop in the Hemoglobin level (Hb is low in hemolytic crises).
How will you diagnose G6PD deficiency?
- There is an increase in the reticulocyte number. These may go as high as 50%.
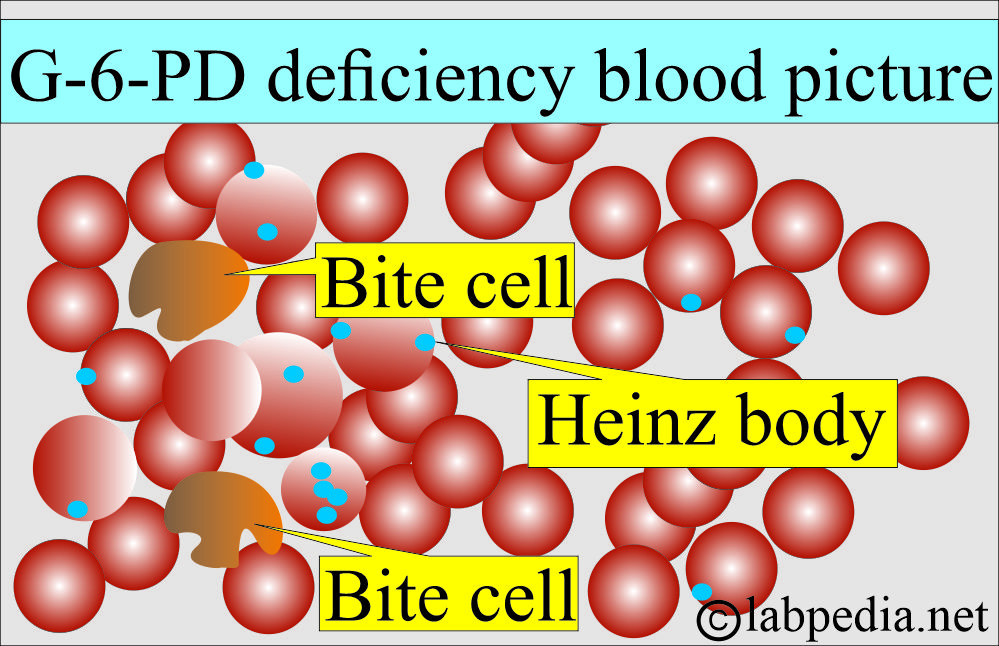
- The peripheral blood smear shows poikilocytosis. During the attack, the peripheral blood smears show:
- RBCs with punched-out defects in their shapes are seen.
- There are fragmented RBCs, and more typically, there are blister cells.
- Normoblastic cells may be seen.
- Spherocytes may be seen.
- Heinz bodies may be found in the RBCs.
- Diagnosed by a screening test for G-6-PD deficiency.
- Screening test = Negative
- Quantitative = 8.0 to 8.86 U/g hemoglobin.
- A definite diagnosis by enzyme assay of RBCs.
- In an acute attack, the screening test is normal.
- However, the screening test will be abnormal in the asymptomatic phase, indicating a deficiency.
- When you give a standard dose of a primaquine tablet, this will be evidenced by the hemolysis as follows:
- Decreasing Hct. It usually starts after 2 to 4 days and peaks in 8 to 12 days.
- Heinz bodies appear in the first week of the dose.
- There is increased serum bilirubin in the first week of the dose, as well as hemolysis.
- Reticulocytes start to increase by the 5th day of the dosage, reaching a maximum of 10 to 20 days.
- Hemolysis subsides spontaneously even if the primaquine is continued.
- The ascorbate cyanide test:
- Add the patient’s RBCs to the ascorbate cyanide.
- G6PD-deficient RBC cells are more sensitive to oxidant stress than normal cells.
- The fluorescent spot test:
- Patient RBCs are incubated with NADP and G6P, measuring NADPH production with fluorescence.
How will you treat G6PD deficiency?
- Stop the offending agent.
- Keep a high urine output.
- Blood transfusion is required if there is severe anemia.
- Babies with G-6-PD deficiency will have neonatal jaundice.
- These babies need phototherapy and maybe a blood transfusion.
What is the prognosis of G6PD deficiency?
- Hemolytic crises in type A are usually self-limited, even if the drug is continued.
- Gallstones may be seen in these patients.
- In crises, Hb may be low; otherwise, it is stable.
- Favism may have a fatal outcome.
What is the warning for patients with G6PD deficiency?
- Do not give sulfonamides or antipyretics in known cases of G6PD deficiency.
Questions and answers:
Question 1: What is the complication of G6PD deficiency?
Question 2: Can you see Heinz bodies in G6PD deficiency?