Albumin (Serum Albumin)
Albumin (Serum Albumin)
What sample is needed for serum Albumin?
- It is done on the patient’s serum.
- How to get good serum?
- Take 3 to 5 ml of blood in a disposable syringe or a vacutainer. Keep the syringe for 15 to 30 minutes at 37 °C and then centrifuge for 2 to 4 minutes to get the clear serum.
- A random sample can be taken.
- You can use a freshly prepared serum or store it at 4 °C, which may keep it stable for more than 72 hours.
What are the precautions for Albumin?
- A fasting sample is preferred.
- Specimens with lipemia or hemolysis should be avoided.
- Avoid prolonged tourniquet. This may increase Albumin and proteins.
- Take into account physical exercise and fever where there is increased filtration.
- Blood samples after the I/V therapy may give low value.
- The drugs that increase the level are anabolic steroids, androgens, corticosteroids, insulin, progesterone, and growth hormone.
- The drugs that can decrease the level are estrogens, hepatotoxic drugs, and oral contraceptives.
What are the indications for serum albumin?
- This test is advised for:
- In liver diseases as a part of a liver panel test.
- Kidney diseases and nephrotic syndrome patients.
- In patients with a severe burn.
- As a part of other tests.
- In a patient suspected of malnutrition.
- In patients where there is a loss from the intestine.
- Patients with cancers, particularly lymphoma and multiple myeloma.
- Albumin is estimated in the third trimester of pregnancy, which may decrease the total protein level.
How will you discuss the pathophysiology of Albumin?
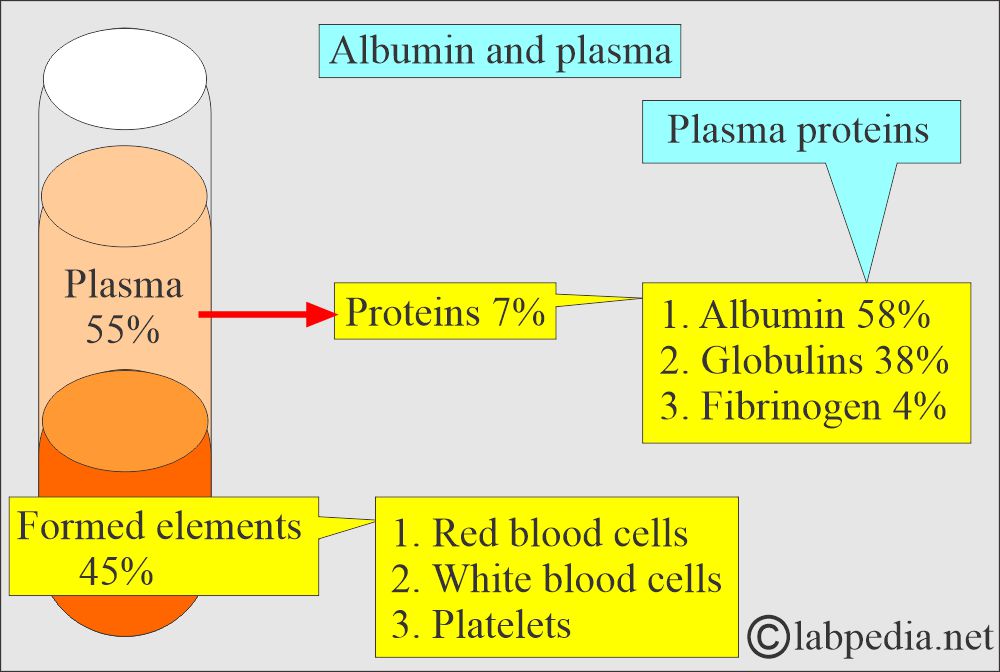
- This is the most abundant protein in the blood.
- Albumin is the most abundant protein in the plasma, constituting 2/3 of total proteins. It is present in around 40% of the plasma and 60% of the extracellular space.
- Plasma proteins are separated into three major groups:
- Fibrinogen (4%).
- Globulins (38%).
- Albumin (58%).
- Total serum proteins are a combination of prealbumin, Albumin, and globulins.
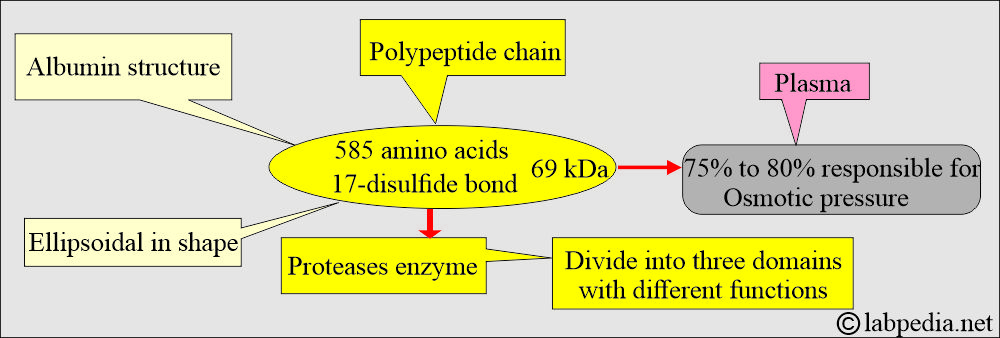
What is the Albumin structure?
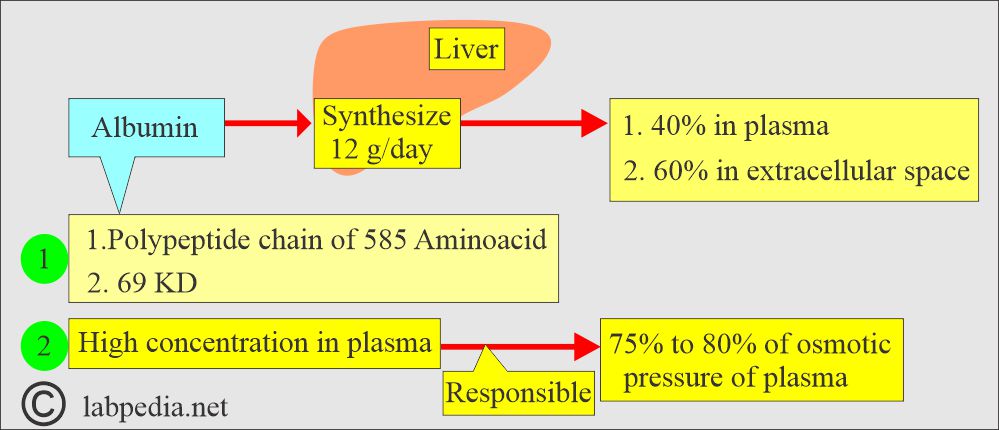
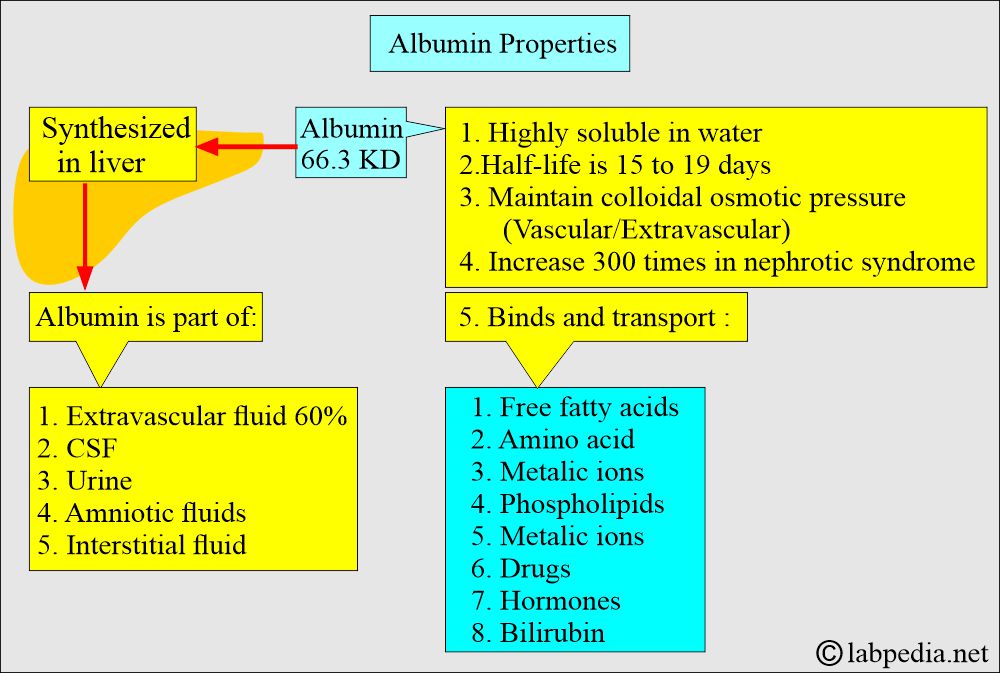
- Albumin is a globular protein with a molecular mass of 66.3 kD.
- Albumin consists of one polypeptide chain of 585 amino acids and contains 17 disulfide bonds.
- Albumin is an anion at pH 7.4 with >200 negative charges per molecule.
- It has no carbohydrate side chains but is highly soluble in water due to its high net negative charge at physiologic pH.
- Albumin can not be stored in the parenchymal cells because of a lack of side Carbohydrate chains.
- It accounts for approximately half of the plasma proteins.
- This is the major protein component of most extravascular body fluids like CSF, urine, amniotic fluid, and interstitial fluid.
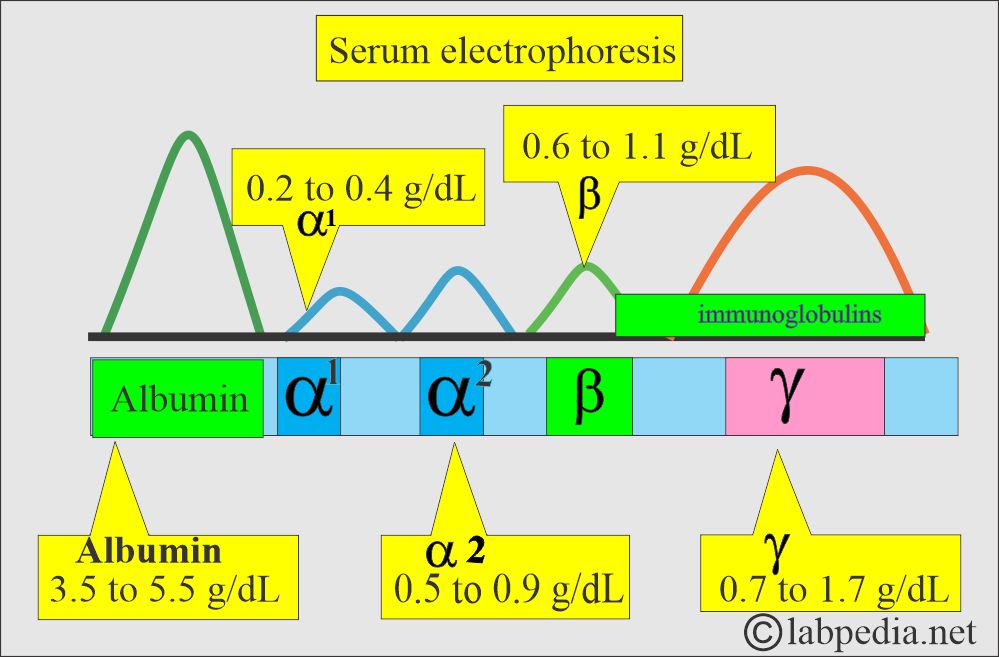
How would you discuss the serum electrophoresis?
- The most common method to separate the proteins is electrophoresis. There are five bands named:
- Albumin.
- It is roughly 60% of the total serum proteins and will migrate farthest toward the anode.
- α1- fraction.
- α2- fraction.
- β- fraction.
- γ- fraction.
- Albumin.
What are the proteins in the blood, cord blood, and serum?
| Type of proteins | Cord blood g/dL | Mother’s serum g/dL | Adult values g/dL |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What is the distribution of the Albumin in the body?
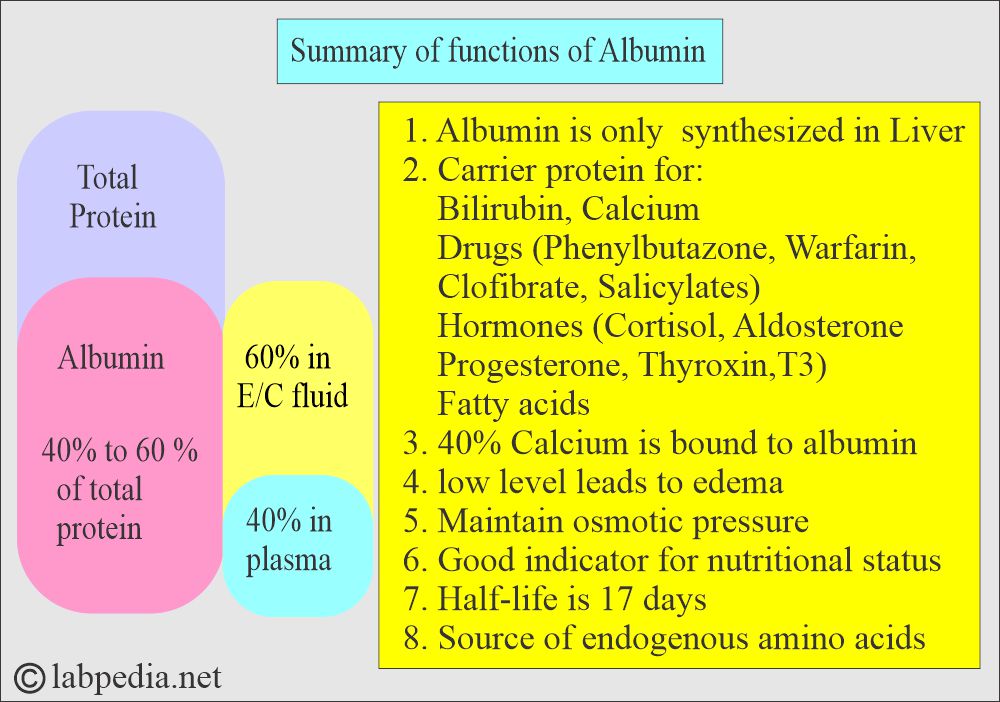
- Albumin makes 40% to 60% of the total proteins.
- There is a high concentration of Albumin in the plasma.
- Its small molecular size is found in most extravascular fluids, CSF, amniotic fluid, urine, and interstitial fluid.
- CSF protein electrophoresis shows albumin around 56% to 76% of the total proteins.
- 40% of the Albumin is present in the plasma, and the other 60% is in the extracellular space.
- Amniotic fluid contains albumin:
- Second trimester = 0.4 g/dL
- At term = 0.9 g/dL
- Around 60% of the Albumin is present in the extravascular space.
- It is highly water-soluble due to its negative charge at normal pH.
- Albumin’s half-life is 15 to 19 days.
- So, hepatic impairment during the albumin synthesis may not have been detected before this period.
How is the synthesis of Albumin?
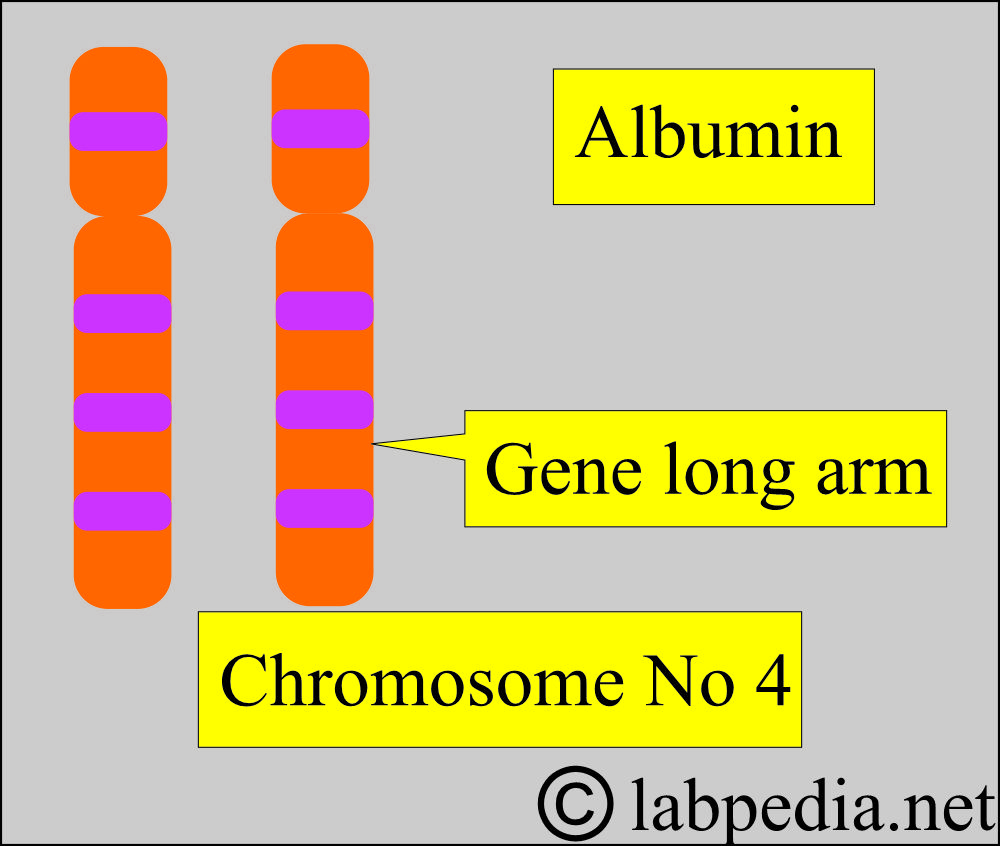
- A gene codes albumin on the long arm of chromosome 4.
- More than 80 genetic variants are reported.
- Albumin synthesis starts at 20 weeks of gestation and continues throughout life.
- During the first 20 weeks of fetal life, α-fetoprotein may serve as the Albumin’s osmotic equivalent.
- The liver produces 12 g of Albumin in 24 hours, representing about 25% of the total protein synthesized by the liver.
- This protein is synthesized primarily from the hepatocytes of the liver.
- It reflects the function of the liver, kidney, or malnutrition.
- The liver’s synthetic reserve is enormous, e.g., 300% or more of the normal rate in nephrotic syndrome.
- Decreased synthesis in the liver is seen in acute or chronic liver diseases, Amyloidosis, malnutrition, and malignancy.
- Dehydration leads to an increase in albumin levels (Hyperalbuminemia).
How the synthetic rate is controlled?
- Colloid osmotic pressure.
- Protein intake.
- Decreased by the inflammatory cytokines.
- The inflammatory cytokines decrease albumin synthesis.
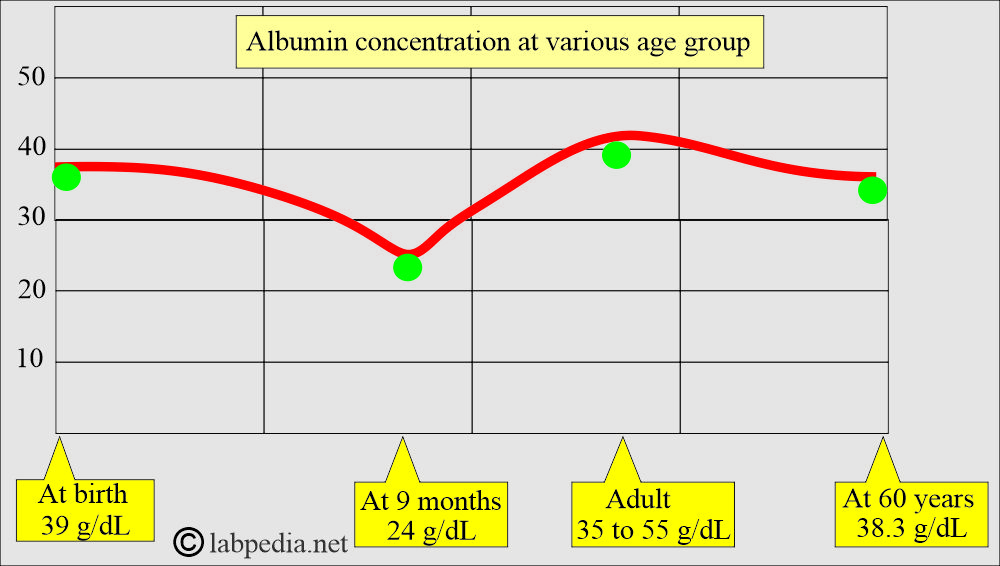
- Albumin Concentration in the Serum:
- At birth, it is 39 g/L, then it decreases to 24 g/L at nine months, again rises to 35 to 55 g/L at adult age, and after 60 years, it is 38.3 g/L.
What is the role of Albumin as a transport protein?
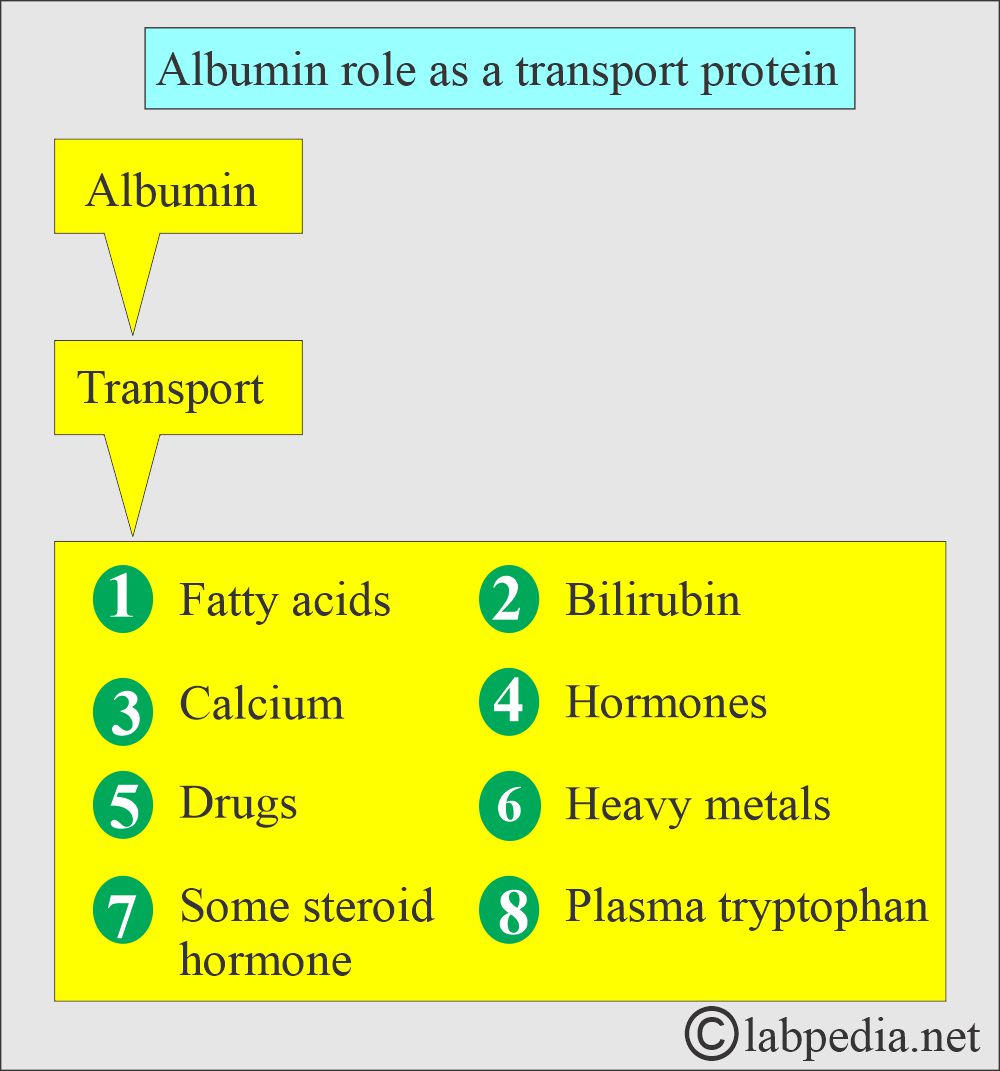
- Albumin binds bilirubin, free fatty acids, calcium, and some drugs.
- Variations in its concentration will markedly affect its role in transporting bilirubin, bile acids, metal ions, and drugs.
- The presence of Albumin in the urine indicates kidney disease.
What is the mechanism of decrease in the albumin synthesis?
- The mechanism for the decrease in serum albumin may be due to decreased synthesis due to:
- Injury to the hepatocytes.
- Decreased protein intake, like malnutrition or starvation.
- If there is impaired absorption of the protein products, such as in sprue,
- Extensive loss of the Albumin seen in:
- In nephrotic syndrome, there is extensive loss of protein in the urine.
- There is a loss of protein in extensive burns or exfoliative dermatitis.
- In protein-losing intestinal diseases (protein-losing enteropathies).
- Shifting the protein in ascites may happen in the liver diseases like cirrhosis.
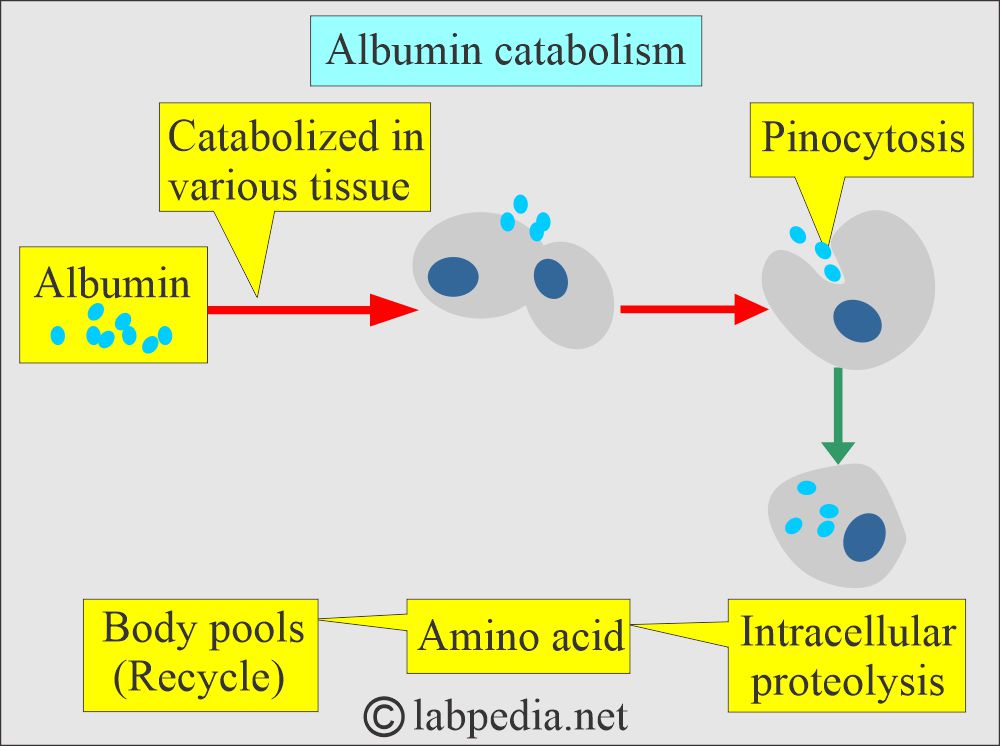
How will you discuss the Albumin catabolism?
- Albumin is catabolized in various tissues.
- It is taken up by the cells as pinocytosis.
- Then, there is proteolysis from the amino acids in the cells, which (amino acids) go into the body pool (recycle).
What are the Albumin functions?
- It is susceptible to liver damage.
- Low albumin results in Edema.
- Albumin is essential for regulating water and solutes’ passage through the capillaries because the albumin molecules are large and don’t diffuse freely through the endothelium.
- Maintaining the osmotic pressure in the blood vessels is needed, without which fluids will leak out.
- One of the most important functions is maintaining the colloid osmotic pressure of the intravascular fluid.
- Because of its high concentration, it is responsible for 75% to 80% of osmotic pressure, which maintains the fluid in the tissues.
- The primary function is maintaining colloidal osmotic pressure in vascular and extravascular spaces with continuous equilibrium.
- Albumin prevents edema.
- Albumin provides nutrition to the tissues and binds various molecules, such as salicylates, fatty acids, magnesium ions, cortisol, hormones, vitamins, and drugs.
- Albumin is a carrier protein for bilirubin, calcium, progesterone, other drugs, hormones, and enzymes.
- Drugs bound to Albumin are sulfonamide, penicillin, aspirin, and dicumarol.
- Albumin is an endogenous source of amino acids.
- Albumin binds and solubilizes nonpolar compounds such as plasma bilirubin and long-chain fatty acids.
- Albumin binds hormones like thyroxine, triiodothyronine, cortisol, and aldosterone.
- 40% of the calcium binds the Albumin.
- Some drugs like phenylbutazone, warfarin, salicylates, and clofibrate are bound tightly to Albumin.
- Low plasma albumin allows water to move out of the vascular bed, leading to edema.
- Albumin is important in the endogenous metabolism of calcium, fatty acids, bilirubin, drugs, and hormones.
What is the albumin/globulin ratio (A/G)?
- It is normally found = >1.0.
- A/G ratio <1.0 is usually seen in liver diseases.
What is the Albumin/Creatinine ratio (ACR)?
- It evaluates patients with Diabetes Mellitus and renal function.
How will you differentiate the Albumin/creatinine ratio and microalbuminuria?
| Clinical parameters | Normal values | Microalbuminuria | Clinical albuminuria |
|
|
|
|
|
|
|
|
How will you discuss the Diabetic Microalbuminuria?
- It is defined when the Albumin excretion in the urine is 20 to 200 µg/min (30 to 300 mg/24 hours of the urine sample).
- These findings are found in at least 2 to 3 samples collected within six months.
- The albumin/creatinine ratio is the first lab test to detect early microalbuminuria on a random urine sample.
- It is calculated as:
- Albumin in mg/creatinine in g.
- Albumin excreted in the urine is measured in µg/min (mg/24 hours), and this is called the Albumin excretion rate (AER).
- Microalbuminuria is significant when AER is 20 to 200 µg/min.
- An albumin/creatinine ratio >30 mg/g suggests an overnight excretion rate (AER) >30 µg/min.
- When 30 to 300 mg of Albumin is excreted in 24 hours of urine, the albumin/creatinine ratio is >3.4 mg/mmol.
- Creatinine in urine is measured in g.
What are the causes of Hyperalbuminemia?
- It is when the albumin level is higher than the normal level.
- This is seen in dehydration.
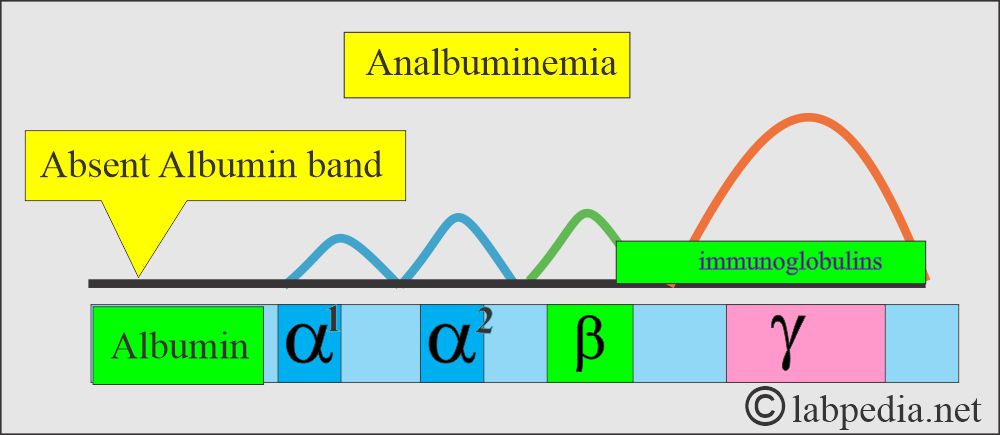
What are the causes of Analbuminemia?
- It is the congenital absence of Albumin.
- These patients are usually asymptomatic or may see occasional mild edema.
- This is a rare autosomal recessive disorder.
- Serum electrophoresis shows a complete absence of the albumin band.
What are the causes of Hypoalbuminemia?
- It is when the albumin level is lower than normal; this may be due to various factors like:
- Impaired syntheses of the Albumin from the liver or decreased intake of the proteins.
- Increased catabolism due to inflammation or tissue damage.
- Malabsorption or malnutrition leads to decreased absorption of amino acids.
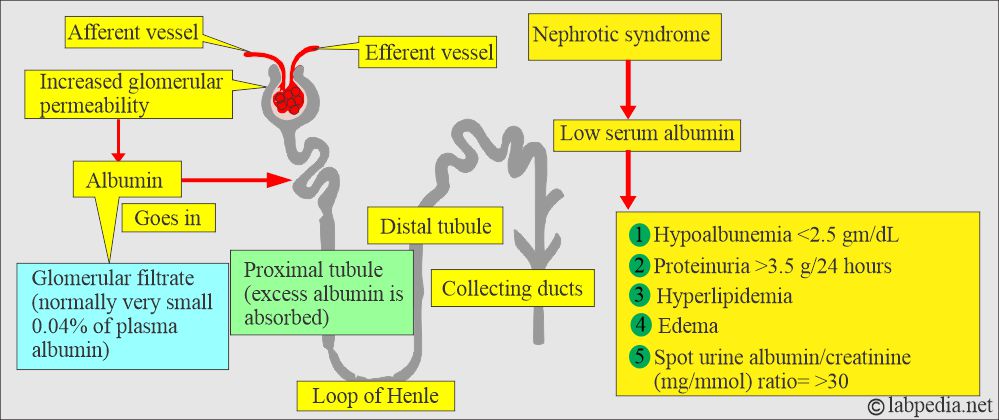
- There is an increased loss of Albumin in the urine in conditions like nephrotic syndrome, chronic glomerulonephritis, diabetes mellitus, and SLE.
- Protein loss in case of burn or protein-losing-enteropathy.
- In the case of ascites, where there is high pressure in the portal system, the Albumin is driven into the peritoneal cavity.
- When the albumin level is <2.0 g/L, it will lead to edema formation. This usually occurs when the albumin loss is through the urine or feces.
- Serum electrophoresis shows a low albumin spike.
What are the normal values of Albumin?
| Type of individuals | Normal range |
|
|
|
|
|
|
| Cerebrospinal fluid (CSF) |
|
|
|
|
|
- Another source: normal albumin values
- Recumbent adult = 3.5 to 5.0 g/dL
- Ambulatory male adult = 4.2 to 5.5 g/dL
- Ambulatory female adult = 3.7 to 5.3 g/dL
- It is lower in the last two trimesters of the pregnancy.
- The level is ∼0.3 g/dL higher in the upright position because of hemoconcentration.
- Reference ranges estimated by nephelometry:
- Newborn 2 to 4 days = 2.8 to 4.4 g/dL
- Adults = 3.4 to 5.0 g/dL
- >60 years = 3.4 to 4.8 g/dL
What are the proteins by serum electrophoresis?
| Fraction of the protein | Normal range | % of the total proteins |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What are the causes of decreased Albumin levels?
- Hypoalbuminemia may take place from one of the following mechanisms:
- Impaired synthesis.
- Increased catabolism.
- Protein loss.
- Reduced absorption of the amino acids.
- Altered distribution of the albumin-like ascites.
- Severe hypoalbuminemia is due to the loss of Albumin in the urine or feces. The level is below two g/L, and edema is usually present.
- Acute and chronic inflammations:
- The causes are hemodilution, extravascular space loss, increased cell consumption, and decreased synthesis.
- Rheumatoid arthritis, granulomatous process, most bacterial infections, vasculitis, ulcerative bowel disease, and certain parasitic infestation.
- Due to decreased synthesis by the liver:
- This may be due to the increased amount of immunoglobulins and loss of Albumin into the extravascular space.
- This may also be due to decreased synthesis because of toxins or alcohol.
- The liver can compensate for Albumin synthesis, which causes approximately 95% of liver function loss.
- In acute and chronic liver diseases, Amyloidosis, Malignancies, Congestive heart disease, and constrictive pericarditis.
- Urinary loss:
- As Albumin is relatively small and globular, a significant amount is filtered into the glomerular urine.
- The majority is then reabsorbed by the proximal tubular cells.
- Normal urine contains 20 mg of Albumin per gram of creatinine.
- Excretion above this level is seen in the following:
- Increased glomerular filtration.
- Tubular damage.
- Hematuria.
- Or a combination of the above factors.
- Examples are:
- In Nephrotic syndrome.
- Thermal burns.
- Trauma and crush injuries.
- Transudation and exudation from any hollow organs.
- Increased loss via body fluids.
- Due to Increased catabolism:
- This leads to decreased albumin-like fever, antimetabolites, thyrotoxicosis, and certain malignancies.
- Due to gastrointestinal loss:
- With the inflammatory disease of GIT.
- Chronic protein-losing enteropathy.
- Due to Increased blood volume (hypervolemia):
- This leads to decreased albumin-like exogenous estrogen therapy, Myeloma, and congestive heart failure.
- The serum level decreases in pregnant ladies.
- The person is on a low-protein diet.
- Albumin is decreased in the following:
- After weight loss surgery.
- Whipple disease.
- Sprue.
- Crohn’s disease.
- Analbuminemia is a rare genetic deficiency where the plasma albumin level is <0.5 g/L.
- Electrophoresis shows no albumin bands.
- Major clinical manifestations are related to abnormal lipid transport. Edema is surprisingly very mild.
- How will you summarize decreased Albumin?
- Inflammations.
- Hepatic diseases.
- Urinary loss.
- Gastrointestinal loss.
- Edema and ascites.
- Protein malnutrition.
What are the causes of increased Albumin levels?
- Naturally, there is no reason for the increase in albumin levels.
- Dehydration or any other cause leading to a decrease in the plasma volume causes an increase in the level.
- High protein diet.
- When the tourniquet is applied for a long time.
What are the causes of Hyperalbuminemia and Hypoalbuminemia?
| Hypoalbuminemia | Hyperalbuminemia |
|
|
Questions and answers:
Question 1: What is the main function of albumin?
Question 2: What is diabetic microalbuminuria?

Very Well Expanied ..
Explain Pattern is so much high , Informative & Easy , that can understand Easily to Everyone..
Nice & Thanks For this..
awesome notes!!
Thanks.
I thank you very much from my heart for this medical information that you have put on the site
Thanks for the comments.