Acid-Base Balance:- Part 4 – Arterial Blood gases (Blood Gases)
Arterial Blood Gases
What sample is needed for arterial blood gases?
- The better choice is the Radial artery.
- You can draw the blood from the femoral artery or brachial.
- Blood can be drawn from the indwelling arterial line.
- The tests are done immediately because oxygen and carbon dioxide are unstable.
- Place the sample on ice and immediately transfer it to the lab.
- Arterial blood is better than venous blood.
- A syringe or tube is filled for venous blood, and a tourniquet is used for a few seconds.
- Arterial blood is risky, and a trained person should do it.
- Never apply a tourniquet.
- Don’t apply the pull to the plunger of the syringe.
What are the indications for Arterial Blood gases?
- This test is done on mostly hospitalized patients.
- Mostly, the patients are on a ventilator or unconscious.
- It monitors critically ill nonventilator patients.
- For patients in pulmonary distress.
- To assess the respiratory (ventilation), metabolic (renal) acid/base, and electrolyte imbalance.
- Its primary use is to monitor arterial blood gases and the pH of the blood.
- Also used to monitor oxygenation.
- Used to qualify a patient for the use of oxygen at home.
- This is used as preoperative baseline parameters.
What are the precautions for the collection of Arterial Blood gases?
- Avoid pain and anxiety in the patient, which will lead to hyperventilation.
- Hyperventilation due to any cause leads to decreased CO2 and increased pH.
- Keep blood cool during transit.
- Don’t clench your finger or fist. This will lead to lower CO2 and increased acid metabolites.
- pCO2 values are lower in the sitting or standing position than in the supine position.
- Don’t delay the performance of the test.
- Avoid air bubbles in the syringe.
- Excess of heparin decreases the pCO2 by maybe 40% less.
- Not mixing the blood properly before the test may give a false result.
- A prolonged tourniquet or muscular activity decreases venous pO2 and pH.
- The best way to collect arterial or venous blood is anaerobic.
- Arterial blood precautions:
- Only syringe and needle, no tourniquet, no pull on the plunger.
- Venous blood precautions:
- The needle and syringe of the heparinized evacuated tube filled, drawn a few seconds after the
tourniquet. - Liquid heparin is the only suitable anticoagulant with the proper amount.
- Less amount will lead to clot formation.
- The increased amount will lead to increased CO2 and a decrease in pH.
- This will lead to a dilutional error.
- Glass collection devices are better than plastic.
- The needle and syringe of the heparinized evacuated tube filled, drawn a few seconds after the
- Avoid in patients with coagulopathy.
- Avoid in a patient with AV fistula.
Venous blood gases (VBG)
- It gives information about the local area from where the blood sample is taken.
- Venous blood color is dark red.
- Metabolism of the extremity varies from area to area.
- In shock, the extremities are cold, and less blood perfusion.
- During the local exercise of the extremities, such as opening and closing the fist with power.
- In case there is an infection in the sample area,
- A blood sample from the central venous catheter is not a good mix of blood from various body parts. For well-mixed blood samples should be taken from the right ventricle or the pulmonary artery, which
is not an easy procedure. - A blood sample from the central venous catheter:
- Shows low O2 concentration, which means that:
- Either the lungs have not oxygenated the arterial blood well.
- Or the Heart is not circulating the blood effectively.
- Shows low O2 concentration, which means that:
Arterial vs. Venous blood
What is the difference between arterial and venous blood?
| Biochemical parameters | Arterial blood | Venous blood |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Arterial blood gases (ABG):
- It gives a good mixture of blood from various areas of the body.
- Arterial blood color is bright red.
- Arterial blood measurement gives a better status of lung oxygenation.
- If arterial O2 concentration is normal, it indicates lung function is normal.
- If mixed venous O2 concentration is low, indicating the heart and circulation are failing.
- Arterial blood gives information about the lung’s ability to regulate the acid-base balance through the
retention or release of CO2.- Can also be checked the effectiveness of the kidneys in maintaining the appropriate bicarbonate
level.
- Can also be checked the effectiveness of the kidneys in maintaining the appropriate bicarbonate
What is the significance of the Arterial blood gases?
- Arterial blood gases are a common test that provides a useful and potentially life-saving treatment.
- The following parameters are measured, and the rest are calculated:
- pH will give us information about the acid-base balance.
- pO2 will tell us about oxygenation (ventilation).
- pCO2 will also tell us about the acid-base balance.
- pH = 7.35 to 7.45.
- pO2 = >80 mm Hg.
- pCO2 = 35 to 45 mm Hg.
- The above three parameters are needed to help the patient’s management.
Arterial blood gases
How will you define arterial blood gases?
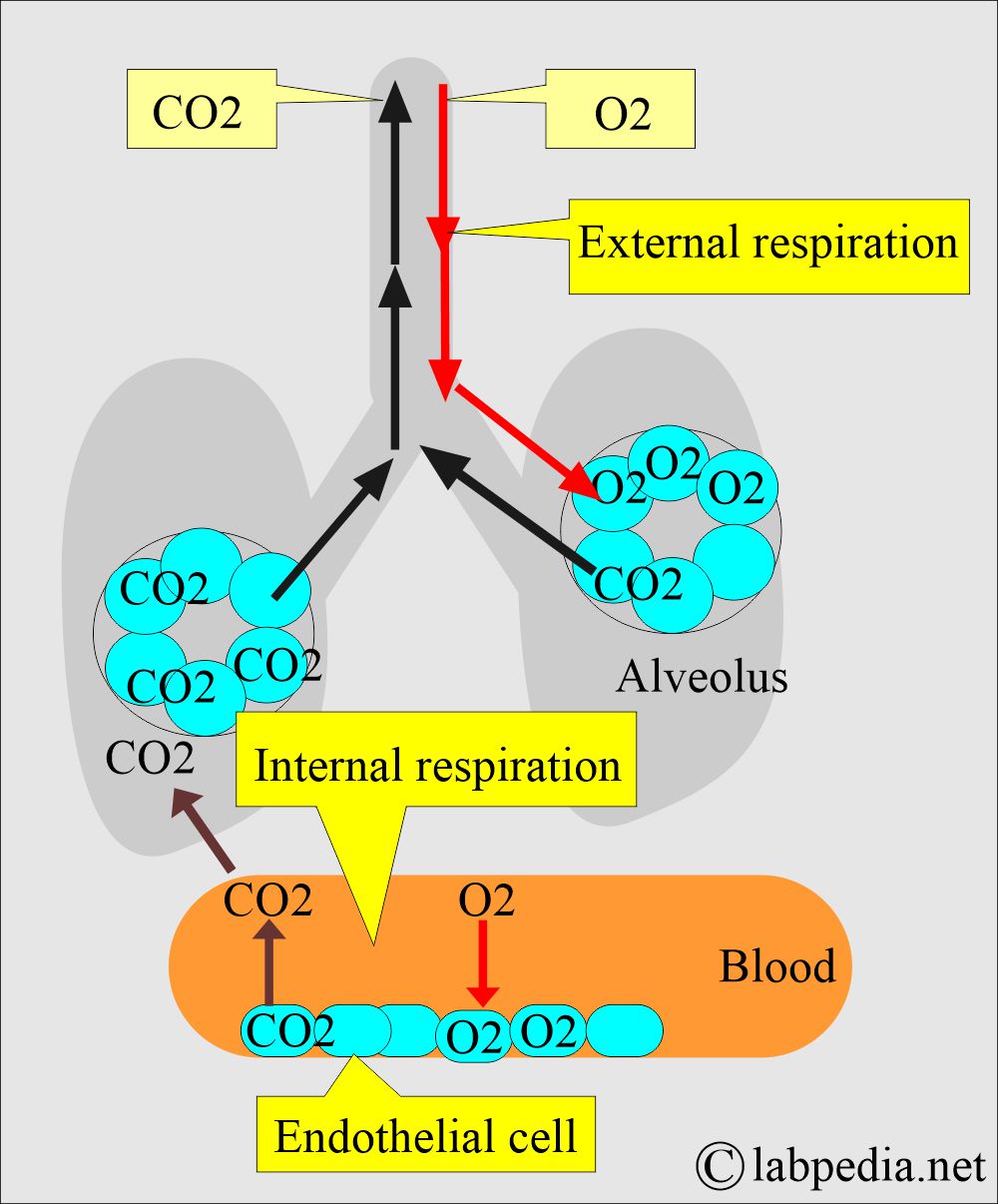
- Blood arterial gases measure the balance between Oxygen (O2) and carbon dioxide (CO2), giving information about the function of the lungs.
- It also measures the acid-base balance in the body.
- Lunga and kidneys work together to keep the acid-base balance.
- Blood arterial gases measure various gases in the arterial blood. It will tell the metabolic and respiratory status.
- Arterial blood gases include:
- The partial pressure of oxygen (paO2).
- Oxygen saturation.
- The partial pressure of carbon dioxide (paCO2).
- Bicarbonate level (HCO3–).
- pH level.
What are the blood arterial gases parameters?
The pH of the blood
- The pH of a solution is the negative logarithm of the hydrogen ion activity.
-
-
- pH = –logaH+.
-
-
- The acid-base status of the body is assessed by:
-
-
- pH.
- pCO2.
-
-
- While blood passes through the lung, O2 moves to the blood, and CO2 enters the lung.
- As the blood hydrogen concentration increases, the pH decreases; if hydrogen ions decrease, the pH increases.
- The decrease of one pH unit represents a 10 times increase in H+ activity.
- The average blood pH of 7.40 is equal to the H+ ions concentration of 40 nmol/L.
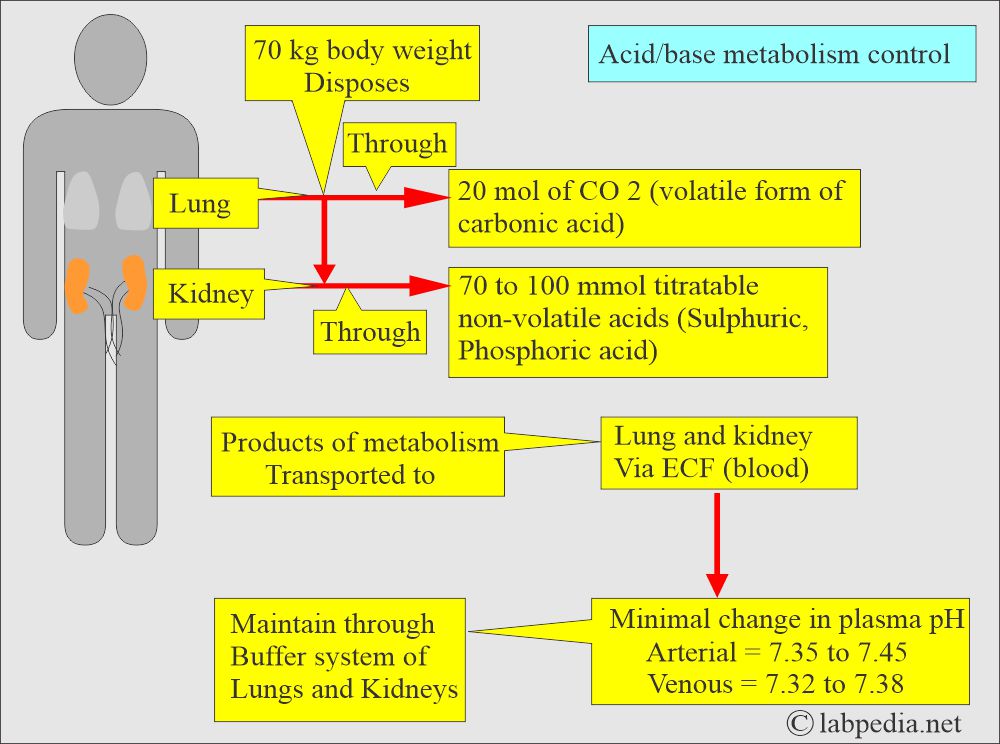
- The lungs and the kidneys regulate the pH of the plasma.
- The acids found in the blood are carbonic acid (H2CO3), dietary acids, keto acid, and lactic acid.
- pH indicates acidity and alkalinity.
- In respiratory or metabolic alkalosis, the pH will be high.
- Respiratory acidosis or metabolic acidosis will decrease the pH value.
- pH alkaline when it is >7.4.
- pH is acidic when it is <7.35.
- Acidemia = pH <7.35
- Alkalemia = pH >7.45
How will you discuss the pCO2 (Partial pressure of the carbon dioxide)?
- pCO2 measures the partial pressure of CO2 gas in the blood (arterial blood, plasma, or serum) measured in mm Hg.
- This is proportional to the amount of dissolved CO2 or the concentration of the CO2.
- pCO2 is a measurement of ventilation capability.
- The units are the same as pO2.
- pCO2 in the blood is 10% in the plasma and 90% carried by the red blood cells.
- With respiration, CO2 is breathed out, and the pCO2 level drops will depend on the breathing rate.
- The faster and more deeply one breaths, the more CO2 is blown off, and the pCO2 level drops.
- pCO2 is the respiratory component in acid-base determination because the lungs control this value.
- As the CO2 level increases, the pH level will decrease.
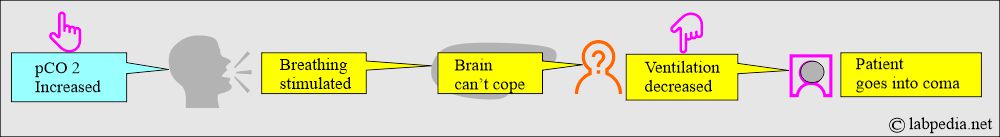
- The pCO2 level in blood and CSF is a major stimulant to the breathing center in the brain.
- As the pCO2 level rises, breathing is stimulated.
- When the brain can not cope with increased pCO2 and cannot blow off excess CO2, the brain is depressed, ventilation decreases, and the patient goes into a coma.
- In metabolic acidosis, the lungs try to compensate by blowing more CO2 to raise pH.
- In metabolic alkalosis, the lungs try to compensate by retaining the CO2 to lower pH.
What is the HCO3 or CO2 content?
What are the methods to measure CO2 contents?
- The total CO2 contents are determined from the heparinized arterial or venous blood drawn anaerobically.
- This can be done in a vacuum tube or a syringe, which is quickly capped.
- The blood is centrifuged, and the plasma is separated.
- Next, plasma is analyzed for CO2 by converting HCO3– and H2CO3 to the gas form.
Write briefly HCO3– and CO2?
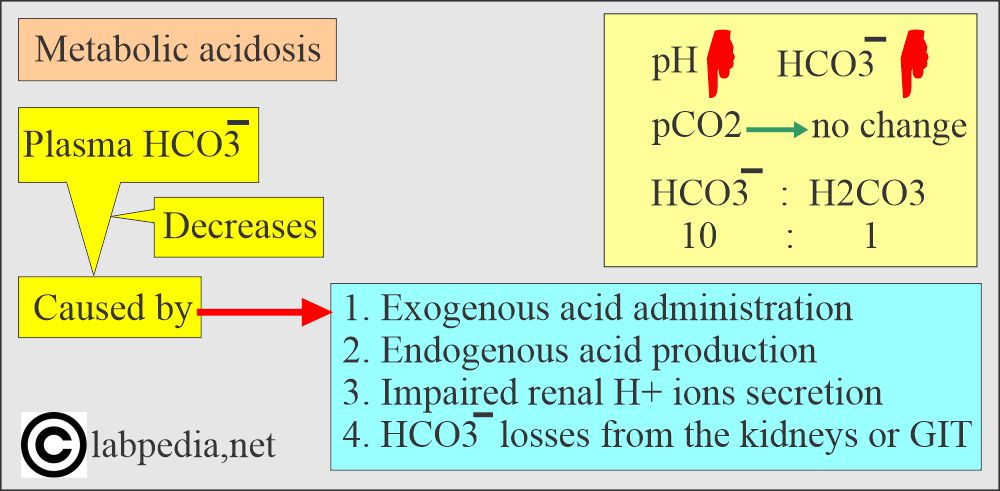
- Most of the CO2 contents are HCO3¯ in the blood because the dissolved amount of the CO2 and H2CO3 contents are very small.
- The HCO3– ions can be measured directly as HCO3– or indirectly by CO 2 contents.
- Total CO2 = HCO3¯ + Dissolved CO2.
- The most important buffer system of the plasma is HCO3¯ / H2CO3.
- It is also present in the RBC but at a lower concentration.
- The ratio of base: acid = 20: 1 in plasma.
- The kidney regulates HCO3¯ ions, and it is the measure of the metabolic (Renal) component of the acid-base balance.
- CO2 contents should not be confused with pCO2.
- CO2 contents are indirectly measured by HCO3¯.
- HCO3¯ : Dissolved CO2 = 25 : 1
- Any change in the above equation leads to a change in the pH.
- As the HCO3¯ level increases, the pH also increases.
Write briefly about the HCO3¯ level?
- In metabolic alkalosis, the HCO3– level is elevated.
- In metabolic acidosis, the HCO3– level is decreased.
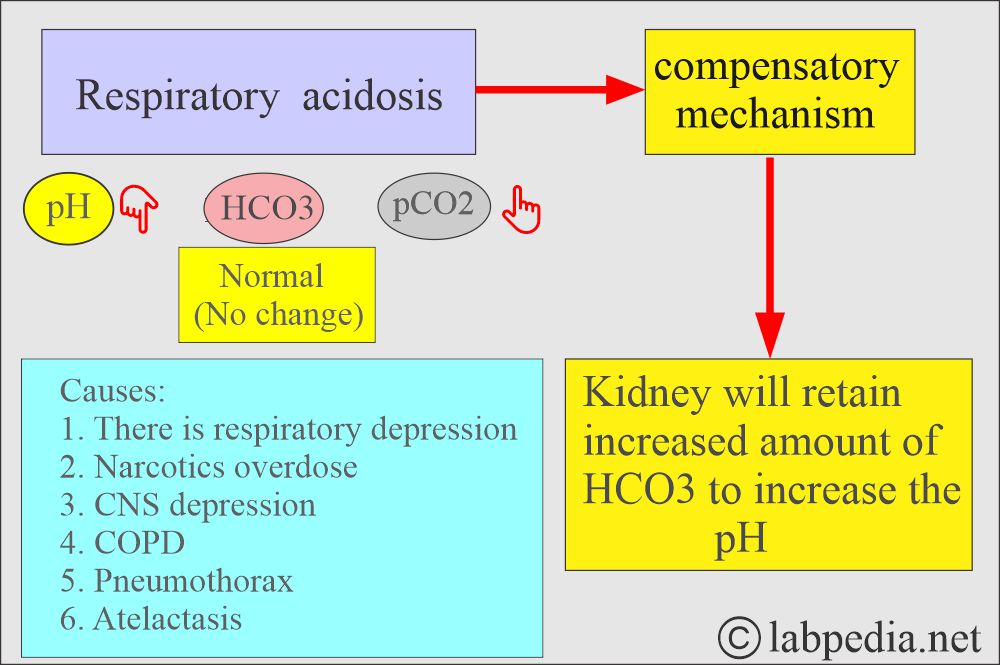
- In respiratory acidosis, kidneys attempt to compensate for increased reabsorption of HCO3¯.
- In respiratory alkalosis, kidneys excrete more HCO3¯ to lower the pH.
What is the role of pH and Bicarbonate in the acid-base system?
| Clinical conditions | pH | Bicarbonate (HCO3) level |
|
|
|
|
|
|
|
|
|
|
|
|
Write briefly about pO2?
- Oxygen in the blood is carried in two forms:
- Dissolved in plasma = <2%.
- Combined with hemoglobin = 98%.
- This partial pressure of the oxygen gas determines the force it exerts in attempting to diffuse through the pulmonary membrane.
- The pO2 reflects the amount of oxygen passing from the pulmonary alveoli to the blood.
- pO2 is the measure of the pressure of O2 present in the plasma.
- pO2 is the indirect measure of O2 contents of arterial blood.
- The pO2 level is decreased in the following ways:
- Pneumonia.
- Shock lung.
- Congestive heart failure.
- Congenital heart diseases.
- Patient under-ventilated.
Write O2 saturation briefly?
- O2 saturation indicates % of hemoglobin saturated with oxygen. OR:
- This measurement is the ratio between the actual O2 content of the hemoglobin and the potential maximum carrying capacity of the hemoglobin.
- O2% saturation is the percentage indicating the relationship between O2 and hemoglobin.
- This is not the O2 content.
- The combined measurement of:
- O2 saturation.
- pO2.
- Hemoglobin.
- This indicates the amount of O2 available to the tissues for oxygenation.
- When 92% to 100% of hemoglobin carries O2, perfusion or oxygen supply to the tissue is normal.
- With the decrease of the pO2 level, the saturation of hemoglobin also decreases.
- The tissues cannot get adequate oxygen when the O2 saturation is 70% or low.
- What are the Precautions for O2?
- Please avoid smoking or exposure to secondhand smoke or CO (carbon monoxide). In such cases, the COHb level increases.
- Avoid the use of paint or varnish.
What is the O2 content?
- The actual amount of O2 in the blood is termed the O2 content.
- Normally, all O2 is bound to hemoglobin.
- About 98% of all O2 delivered to the tissue is transported in combination with the hemoglobin.
- The following formula calculates O2 contents:
-
- O2 content = O2 saturation x Hb x 1.34 + pO2 × 0.003
-
What are the normal electrolytes and blood gases?
Source 1
pH
- Adult / child = 7.35 to 7.45
- Newborn = 7.32 to 7.49
- 2 months to 2 years = 7.34 to 7.46
- pH venous blood = 7.31 to 7.41
Body fluids pH - Arterial blood
- 7.38 to 7.42
- Venous blood
- 7.37
- Cerebrospinal fluid
- 7.32
- Pancreatic fluid
- 7.8 to 8.0
- Gastric juice
- 1.0 to 3.0
- Urine
- 5 .0 to 6.0
pCO2
- Adult /child = 35 to 45 mm Hg
- Child <2 years = 26 to 41 mm Hg
- Venous blood = 40 to 50 mm Hg
HCO3
- Adult / child = 21 to 28 mEq/L
- Newborn / infants =16 to 24 mEq/L
pO2
- Adult/child = 80 to 100 mm Hg
- Newborn = 60 to 70 mm Hg
- Venous blood = 40 to 50 mm Hg
O2 saturation
- Adult/child = 95 to 100%
- Old people = 95%
- Newborn = 40 to 90%
O2 content
- Arterial blood = 15 to 22 vol%
- Venous blood = 11 to 16 vol%
Source 2
| Chemicals | Arterial | Venous | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
Source 3
Normal Values of Analytes
| Lab test | Blood | Venous | Arterial |
|
|
|
|
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
|
|
|
|
What are the Arterial blood gases?
| Biochemical parameter | Adult | Pediatric group |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What are the interpretations of and role of arterial gases in the acid-base balance?
Normal picture = pH normal, PCO2 normal, HCO3 normal.
- Acidemia means arterial blood pH <7.4.
- Acidosis means a systemic increase in H+ ions.
- Alkalemia means arterial blood pH >7.4.
- Alkalosis means a systemic decrease in H+ ions.
What are the findings of Respiratory acidosis?
- pH and HCO3– go in the opposite direction.
- pH lower, pCO2 high, HCO3– high.
- Seen in respiratory depression due to any cause.
- Hypoventilation.
- Excessive retention of CO2.
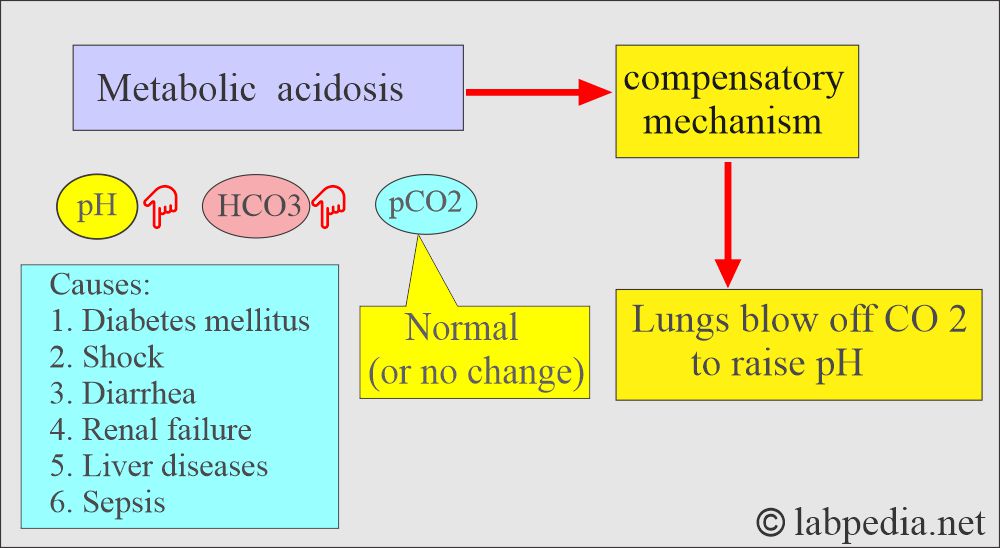
What are the findings of metabolic acidosis?
- pH and HCO3– go in the same direction.
- pH low, pCO2 low, HCO3– low.
- Seen in diabetes, shock, renal failure, and an intestinal fistula.
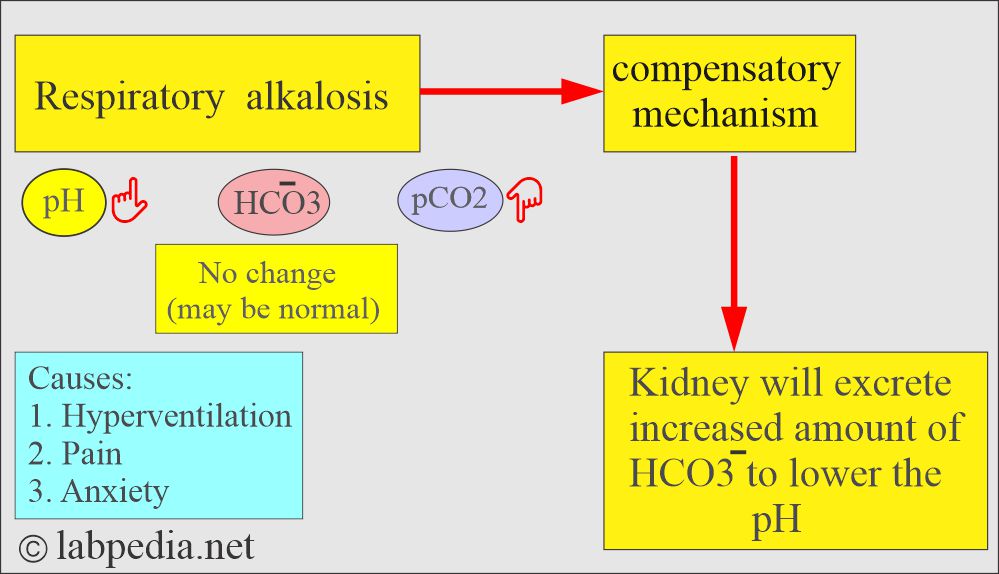
What are the findings of Respiratory alkalosis?
- pH and HCO3– go in the opposite direction.
- pH is high.
- pCO2 low.
- HCO3– is normal or slightly decreased.
- Seen in hyperventilation.
- Excessive loss of CO2.
What are the findings of Metabolic Alkalosis?
- pH and HCO3– go in the same direction.
- HCO3 is >30 meq/L.
- pH high, pCO2 high, HCO3– high.
- Urine pH >7.0 (Unless there is severe hypokalemia).
- Serum K is usually low.
- Seen in sodium bicarbonate overdose, prolonged vomiting, and nasogastric drainage.
Interpretation of the various parameters:
Urine pH:
- pH = < than 7.4 = acidosis.
- pH = > than 7.4 = alkalosis.
pCO2
- pCO2 is high = It is respiratory acidosis.
- pCO2 is low = It is metabolic acidosis.
- pCO2 is low = It is respiratory alkalosis.
- pCO2 is high = It is metabolic alkalosis.
HCO3–
- HCO3– high = It is in respiratory acidosis.
- HCO3– low = It is in metabolic acidosis.
- HCO3– low = It is in respiratory alkalosis.
- HCO3– high = It is in metabolic alkalosis.
Anion gap
Calculation of Anion gap = Na (140 ) + K (4) — Cl (110 ) + HCO3(24) = 10 meq/L
-
- Normal anion gap = 10 to 12 meq/L = < 12
Table showing the values of pH, HCO3, pCO2, and etiology:
| Clinical condition | pH | HCO3 | pCO2 | Etiology |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What are the critical values?
| Biochemical parameter | Less than | More than |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What is the result of acid-base system changes?
- Acidosis leads to coma and death due to depression in the CNS.
- Alkalosis leads to irritability, tetany, and possible death due to the stimulation of the CNS.
- The acidosis state is more threatening than alkalosis.
How will you summarize the parameters needed for the acid-base balance?
| Lab test | Importance |
|
This will tell:
|
|
This is the partial pressure of CO2, and it will tell:
|
|
This is the partial pressure of the O2 in the arterial blood and tells:
|
Questions and answers:
Question 1: What is the value of pH?
Question 2: What is the critical value of pH?

I agree with you
I agree with you