Acid-base Balance:- Part 1 A – Introduction to the Acid-Base Balance
Acid-base Balance
What sample is needed for acid-base studies?
- pH and blood gases preferably should be done on arterial blood.
- Venous blood is not suitable for judging oxygenation.
- Venous blood can be used to check acid-base status.
- Collect blood for electrolytes at the same time.
- Blood gases, electrolytes, and pH should be performed on blood specimens obtained at the same time because of the variation in labile blood gases.
What are the Precautions for acid-base studies?
- The blood sample should be ice-packed immediately.
- A delay of a few minutes will give false values.
How will you define acid-base balance?
- Acid-base balance refers to the regulation of the pH level in the blood.
- Its main purpose is to maintain homeostasis.
- The ideal pH range for the body is 7.35 to 7.45 in the arterial blood.
- An acid-base balance is essential for life.
What is the mechanism of Acid-base balance and electrolyte regulation?
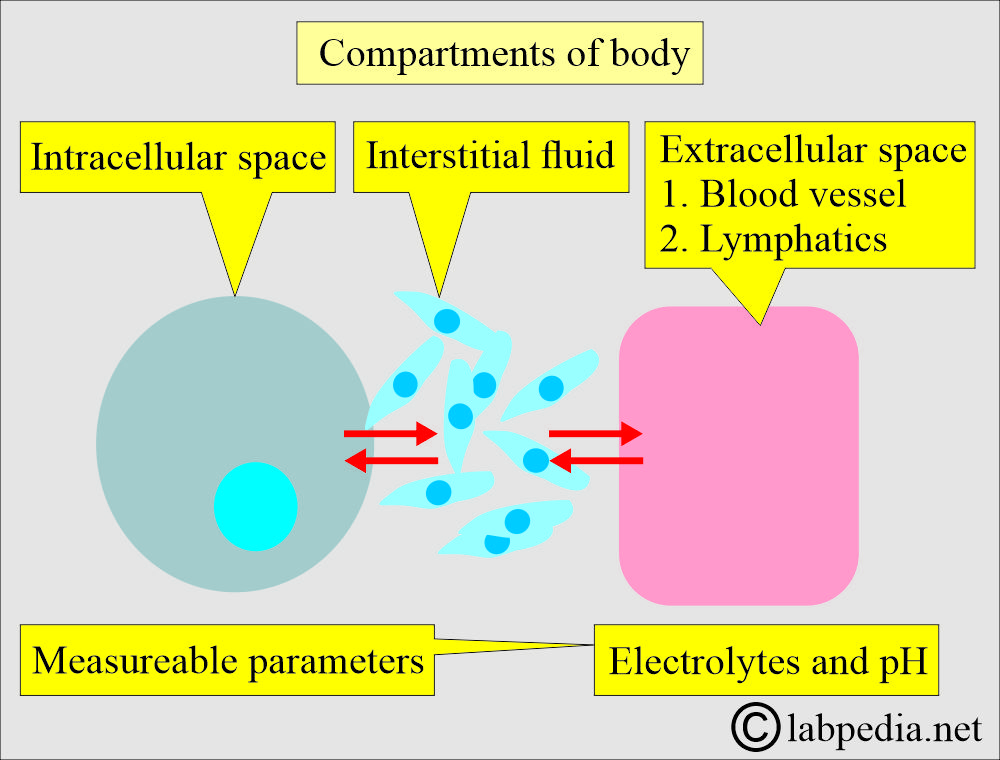
- Electrolytes in different compartments are present in our body.
- Our body has three compartments, and these communicate with each other.
- Intracellular fluid.
- Extracellular fluid (Vascular fluid containing blood and lymph).
- Interstitial fluid.
- Electrolytes and fluids continuously move among these compartments to maintain:
- Homeostasis.
- Cell metabolism.
- Organ functions.
- The body maintains an acid-base balance through the following three systems.
- Buffer system. The bicarbonate, protein, and phosphate buffer systems respond immediately.
- Respiratory system. Lungs regulate CO2.
- Renal system. Kidneys regulate HCO3– and H+ ions.
- The regulation of intracellular and extracellular electrolyte concentration depends on the following factors:
- There is a balance between the intake of substances in a diet containing electrolytes and the electrolytes’ output in feces, urine, and sweating.
- It also depends upon the transport of fluids and electrolytes between ECF and ICF.
- Physiological changes in the concentration of H+ ions in the blood lead to acid-base balance
What factors are important for acid-base balance?
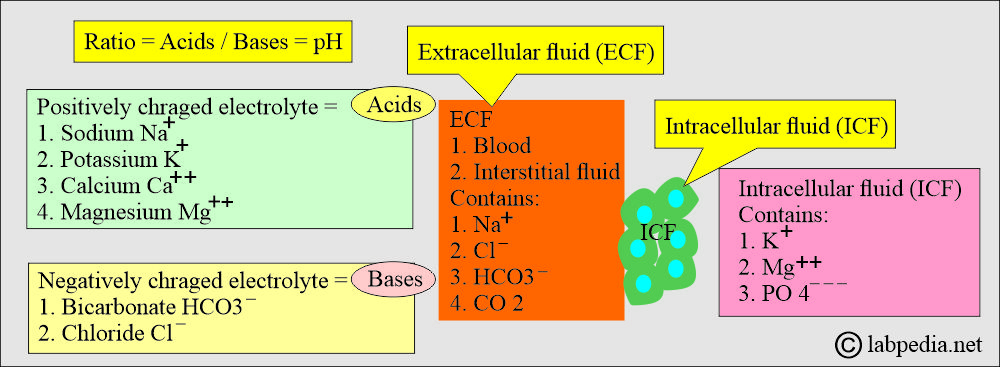
- This regulation of the extracellular fluid environment involves the ratio of acid to base, measured clinically as pH.
- Physiologically, all positively charged ions are called acids, and all negatively charged ions are called bases.
What is the distribution of various anions/cations in body fluids?
| Anions | Extracellular fluid (ECF) | Intracellular fluid (ICF) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cations | ||
|
|
|
|
|
|
|
|
|
|
|
|
What are the possibilities of an acid-base imbalance?
How will you discuss Acidosis (Acidemia)?
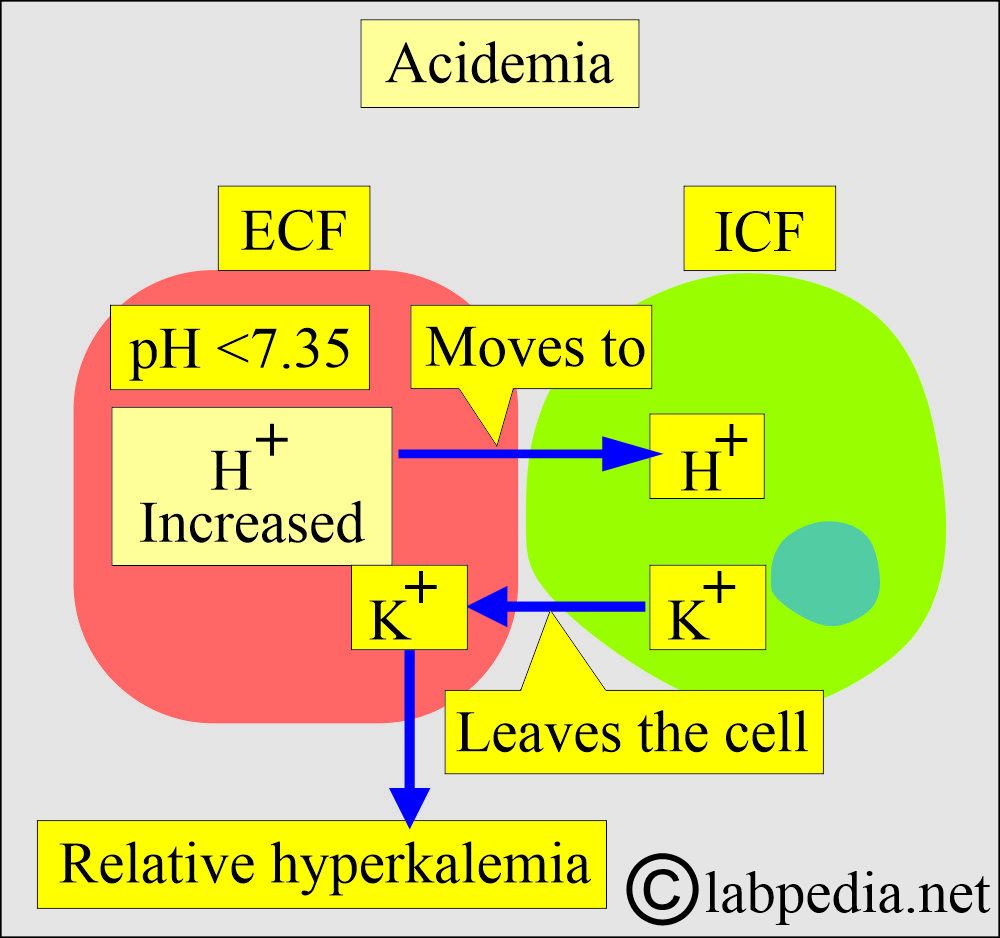
- A systemic increase in the concentration of H+ ions is called acidosis (acidemia).
- Metabolic acidemia:
- Due to metabolic disorders, there is an excess accumulation of acids, such as lactic acidosis, ketoacidosis, and organic acids.
- Respiratory academia:
- It occurs when there is excess CO2, leading to carbonic acid.
- It occurs in chronic obstructive pulmonary disease, pneumonia, and severe asthma.
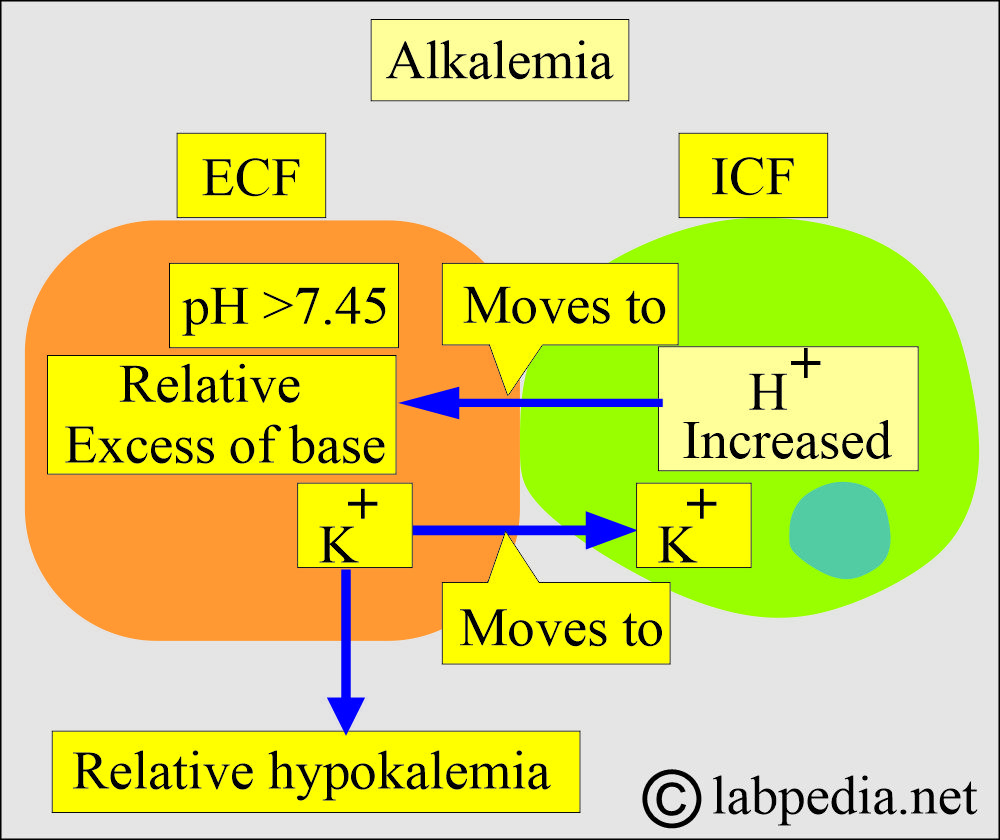
How will you discuss Alkalosis (Alkalemia)?
- A systemic decrease in the H+ ions is called alkalosis (alkalemia).
- Metabolic alkalemia:
- It occurs when there is an excess of HCO3/ or a decrease in non-carbonic acid.
- It may be seen in severe vomiting.
- Excessive intake of alkaline substances.
- In some kidney diseases.
- Respiratory alkalemia:
- There is a decrease in the CO2 in the blood, leading to a decrease in carbonic acid.
- It is seen in hyperventilation, leading to excessive elimination of CO2.
- The body must regulate the acid-base balance within a narrow range to function normally.
- A very slight change in the pH will affect the body.
- A slight change in the H+ ions can change the cells and tissues.
- The pH range is very narrow for normal body functions and needs to be maintained within these limits.
What is the significance of pH in our lives?
| pH value | Effects on the body |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What is the pH of arterial/venous/blood and body fluids?
| Body fluids | pH range | Explanation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What is the Importance of H+ ions?
- To maintain the integrity of the membrane.
- Speed of the metabolic reactions.
- Any change in the pH will lead to more harmful effects than other diseases.
- The symbol pH represents the power of H+.
- When the pH changes by one unit, like 7.0 to 6.0, = [H+] [H+] = the concentration of H+ ions changes 10-fold.
What is the mechanism of body acid formation?
- Metabolism of proteins.
- Metabolism of Carbohydrates.
- Metabolism of fats.
- This must be balanced by the number of basic substances in the body to maintain the normal pH.
- The lungs, kidneys, and bones are the major organs involved in regulating the acid-base balance.
What are the types of Body acids?
- Volatile acids:
- Carbonic acid (H2CO3) is a weak acid that does not easily release the H+ ions.
- The presence of carbonic anhydrase enzyme can eliminate CO2 gas and water H2O.
- CO2 is eliminated through the lungs.
- Nonvolatile acids:
- These are sulfuric acid, phosphoric acid, and other organic acids eliminated through the kidneys.
- These are strong acids and readily give up their H+ ions.
- Nonvolatile acids are secreted into the urine by the renal tubules.
- These acids are about 150 meq/L of H+ ions per day or about one meq/kg body weight.
How will you discuss in detail the buffer systems of the acid-base balance?
- The buffer systems become active in response to changes in the body’s pH as an acid-base balance.
What are the functions of the buffer system?
- Prevent significant changes in pH.
- A buffer can absorb the excess of the H+ ions (acid).
- The buffer system can absorb OH– ions and hydroxide (base).
- The buffer system is present in the intracellular fluid (ICF) and extracellular fluid (ECF).
- The most common buffer systems are:
- Carbonic acid-bicarbonate system.
- Hemoglobin-oxyhemoglobin system.
- Phosphate and protein are the most important intracellular buffers (ICF).
How will you summarize the different buffer systems in acid-base balance?
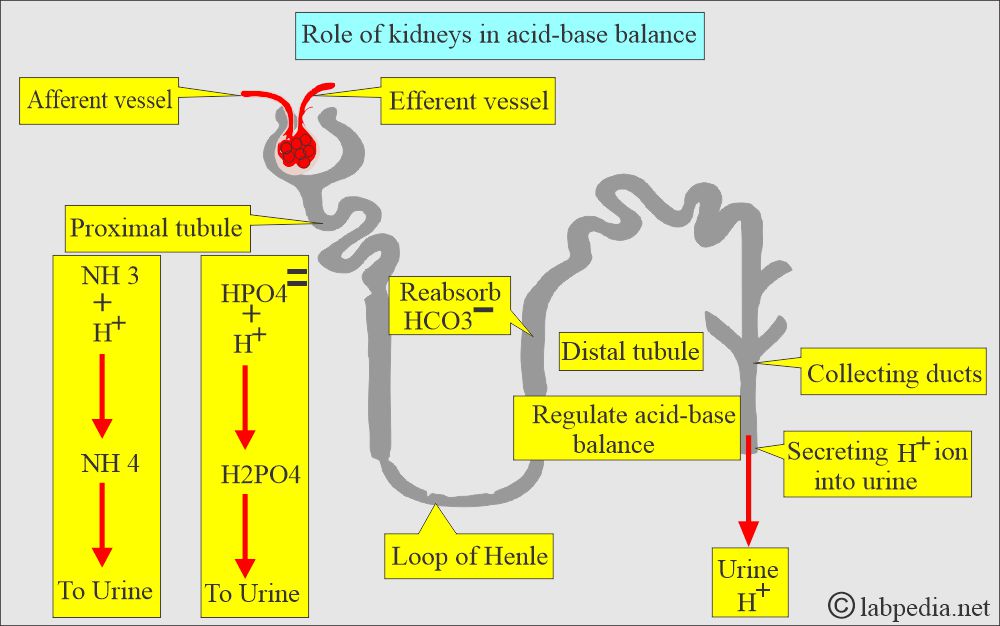
How will you discuss the Renal buffering system?
- The distle tubule of the kidneys regulates acid-base balance by secreting the H+ ions in the urine and reabsorbing the HCO3–.
- Dibasic phosphate (HPO4—) and ammonia (NH3) are two important renal buffers.
- The renal buffering of H+ ions requires CO2 and water (H2O) to form H2CO3.
- The enzyme carbonic anhydrase catalyzes the reaction.
- H+ ions are secreted from the tubular cells and buffer in the lumen by PO4— and NH3 = H2PO–3 + NH4+.
- The rest of HCO3– is reabsorbed.
- If a respiratory Disease causes acidosis or alkalosis, the kidney becomes active and changes H+ and HCO3– ions to return the pH to normal.
- Renal compensation starts in hours to days after respiratory alteration in the pH.
- There is a delay, but the renal buffer system is powerful.
- In academia (acidosis): The kidney excretes an excess of the H+ ions, and these may combine with PO4 (phosphate) or NH3 (ammonia) to form titratable acids in the urine.
- The net outcome is raising the HCO3- ions’ concentration in the ECF and restoring the acid-base balance.
- In alkalemia (alkalosis): kidneys excrete HCO3– ions, usually with Na+ ions.
- The net result is decreased concentration of HCO3- ions in the ECF, and the acid-base balance is restored.
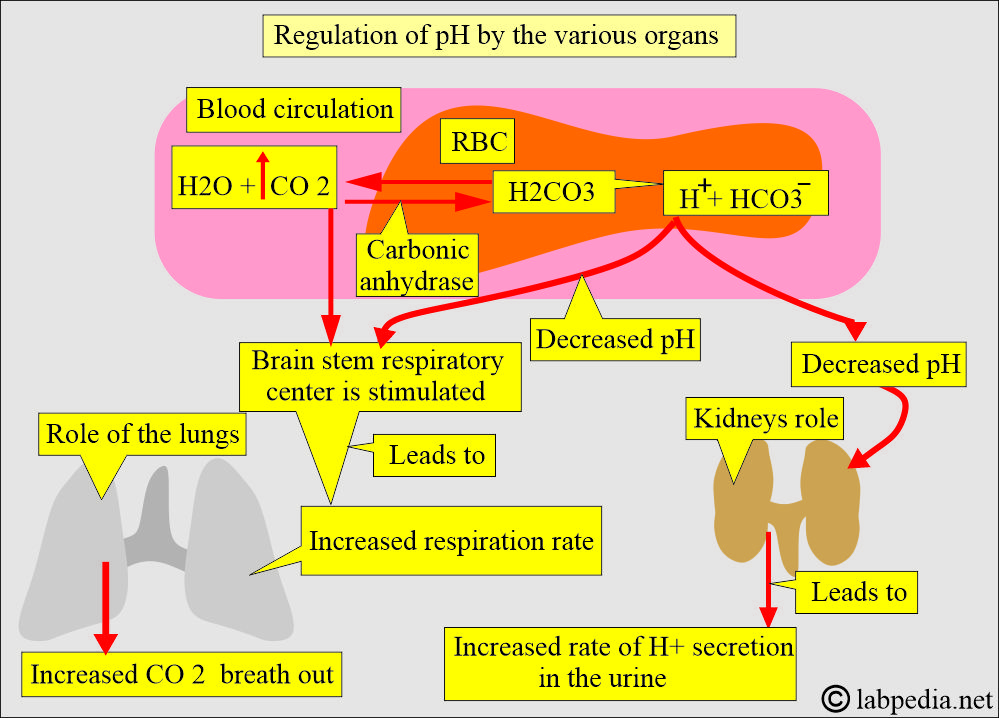
How will you discuss the carbonic acid-bicarbonate buffering system?
- This buffer system operates both in the lungs and kidneys.
- This is the major extracellular buffer system.
- Lungs can decrease the carbonic acid by blowing out the CO2 and leaving water behind.
- Kidneys can reabsorb HCO3– or regenerate new HCO3– from CO2 and water.
- Normal bicarbonate (24 meq/L) and normal carbonic acid (1.2 meq/L) produce a 20:1 relation and maintain a pH of 7.4.
- Both systems are very efficient because:
- HCO3– is easily reabsorbed or regenerated by the kidneys.
- The lungs adjust acid concentration.
- Compensation for the pH is done as follows:
- The respiratory system compensates for pH by decreasing or increasing CO2 by changing the respiration rate.
- The renal system produces more acidic or alkaline urine.
How will you discuss the protein buffering system?
- Hemoglobin (Hb) is the best intracellular buffer system, and it combines with H+ and forms HHb and CO2, forming the HHbCO2 complex.
- When Hb combines with H+ ions, it becomes a weak acid.
- Venous blood Hb is a better buffer system than arterial blood Hb.
How will you discuss the pulmonary role in the acid-base balance?
- In case of acidosis or alkalosis resulting from metabolic or renal diseases, the respiratory system regulates the respiratory rate to restore the pH to normal.
- In acidemia (acidosis), there is an increased respiratory rate and depth to eliminate CO2.
- In alkalemia (alkalosis), there is a decreased respiratory rate and depth to retain the CO2.
What are the various Buffer Systems and their role in the acid-base system?
| Buffer system (pairs) | Buffer system anions | Buffer reaction | Mechanism |
|
|
|
|
(Carbonic acid/bicarbonate buffer system) |
|
|
|
|
|
|
|
|
|
|
|
What are various organs’ roles as a buffer and in acid-base balance?
| Role of organs | Mechanism of these organs’ role in acid-base balance |
|
|
|
|
|
|
|
|
What is the role of various anions in the acid-base balance?
| Anions of acid-base balance | Signs and symptoms | Diagnostic test |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What are the Panic values?
| Clinical parameter | Panic value |
|
|
|
|
|
|
What are the parameters needed for the acid-base balance?
| Lab test | Importance |
|
This will tell:
|
|
This is the partial pressure of CO2, and it will tell:
|
|
This is the partial pressure of the O2 in the arterial blood and tells:
|
Questions and answers:
Question 1: What is acidemia?
Question 2: What is alkalemia?

thanks
Welcome.
Thanks
You are welcome.