Urine pH and Its Significance
Urine pH and its Significance
What urine sample is needed for urine pH?
- The test sample is urine.
- A fresh urine sample is preferred.
What are the indications for urine pH?
- Urine pH tells the systemic acid-base disorder:
- Is it metabolic?
- Or Respiratory.
- Renal tubular acidosis.
- pH is used to identify the type of crystals.
- Urinary pH is very important in the treatment of renal stones and crystals.
- pH is important in managing diseases, bacteriuria, renal calculi, and drug therapy.
How would you discuss the pathophysiology of urine pH?
- The small changes in the urine pH are of little significance.
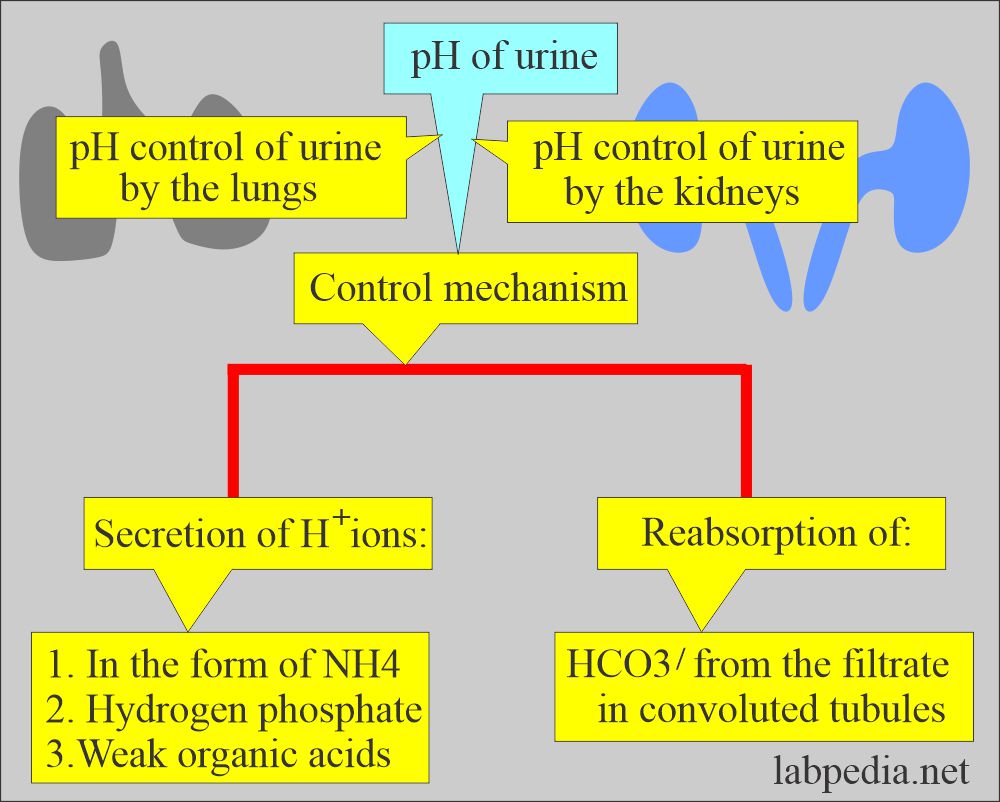
- The lungs and the kidneys are the major regulators of the acid-base content in the body.
- The lungs and kidneys maintain the pH and acid-base balance by secreting H+ ions in ammonium ions, hydrogen phosphate, and weak organic acid and reabsorbing bicarbonate from the filtrate in the convoluted tubules.
- A healthy individual will produce morning urine as acidic with a pH of 5.0 to 6.0.
- Alkaline urine may be found after meals (alkaline tide).
- The random pH ranges from 4.5 to 8.0.
- pH measures the concentration of urine’s free hydrogen (H+) ions.
- Urine pH:
- 7.0 = neutral
- < 7.0 = acidic.
- > 7.0 = alkaline.
- Urine pH indicates the renal tubule’s ability:
- To maintain normal H+ concentration in the plasma.
- To maintain the extracellular H+ concentration.
What is the mechanism to control urine pH?
- The kidneys are the major regulator of the acid-base content in the body.
- Renal distal tubules are the most active site for homeostasis for:
- Plasma electrolyte balance.
- Plasma acid-base balance.
- In the distal tubules, there is a combination of secretion and reabsorption of:
- Na+
- K +
- H+
- In the distal tubules, there is an exchange of H+ for Na+.
- Kidneys have the main function of producing alkaline or acidic urine and maintaining constant body pH.
- Kidneys maintain acid-base by absorption of Na+ and excretion of H+ and ammonia.
What is the significance of urine pH?
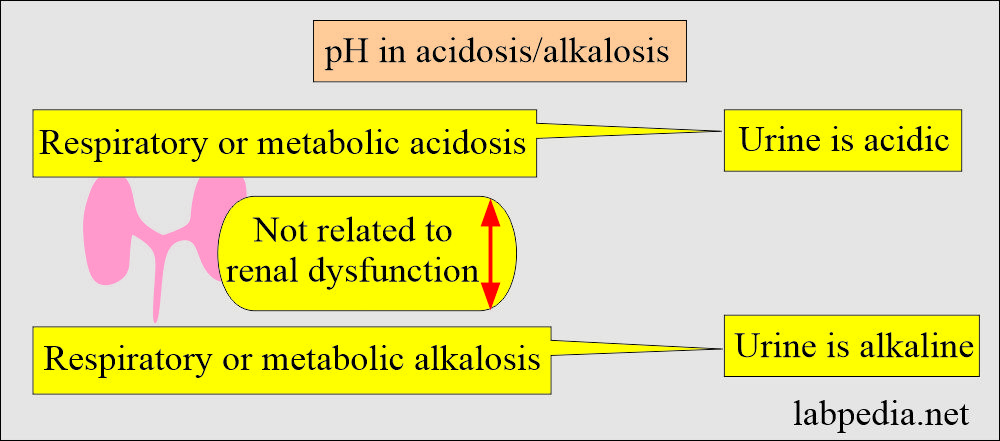
- When there is no renal dysfunction, the urine will be acidic in respiratory or metabolic acidosis.
- In the case of respiratory or metabolic alkalosis, urine will be alkaline when there is no renal dysfunction.
What is the Hydrogen ion secretion and reabsorption in the kidney?
| Hydrogen ions | Prox. tubule | Loop of Henle | Collecting ducts |
| Secretion | + | + | + |
| Reabsorption | – | – | + |
What are the causes of alkaline and acidic urine??
| Alkaline urine (causative agents) | Acidic urine (causative agents) |
|
|
What is the normal urine pH?
Source 2
- Normal pH varies from 4.6 to 8.0 (average 6.0).
How will you measure the urine pH?
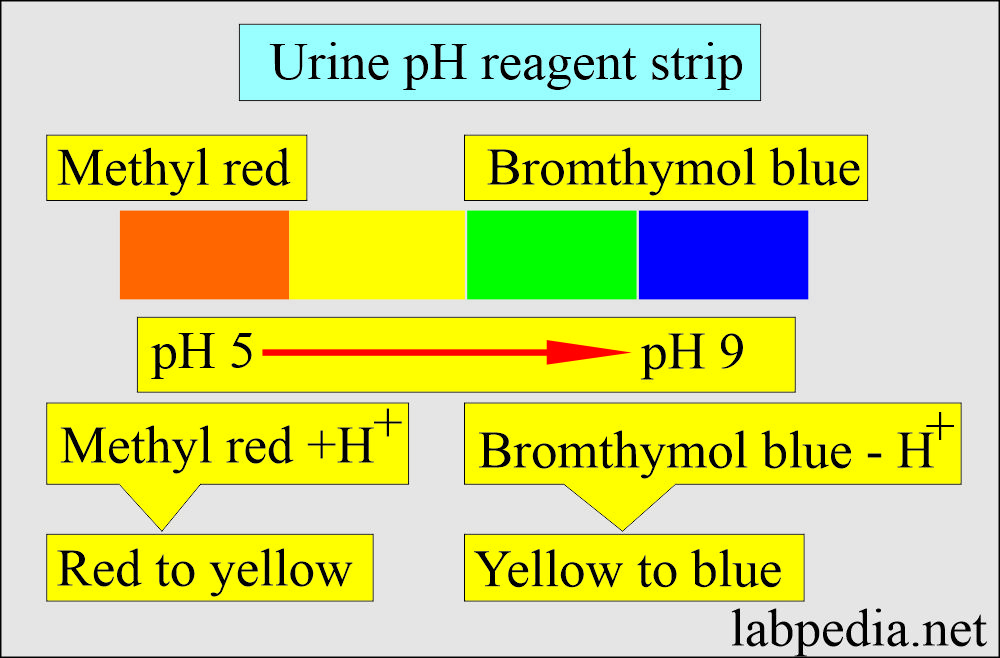
- Test strips were impregnated with the indicator.
- Methyl red in urine pH below 4.2 is red.
- pH above 6.2 is yellow.
- Bromthymol blue is yellow at a pH value below 6.0.
- Blue at the value above 7.6.
- In between the pH, there are shades of orange and green.
- Read after 60 seconds and compare the colors with the given color chart.
- PH 5 is orange, and pH 8.5 is blue.
-
pH electrodes:
- These are more accurate.
What are the causes of high pH >7.0 in urine?
- It Indicates Alkaline urine, maybe due to the following:
- Gastric suction
- Renal Failure
- Vomiting (metabolic acidosis)
- Respiratory alkalosis.
- Hyperventilation.
- Potassium depletion.
- A bacterial infection like Proteus and Pseudomonas.
What are the causes of low pH <7.0 in urine?
- It indicates Acidic urine, maybe due to the following:
- Metabolic acidosis.
- A bacterial infection like E.coli.
- Renal tuberculosis.
- Fever.
- Diabetic ketosis.
- Diarrhea.
- Starvation.
- Uremia.
What is the role of urine pH in renal calculi?
- The renal stone formation depends upon urine pH.
- Calcium phosphate, calcium carbonate, and magnesium phosphate stones form in alkaline pH, so keep the urine acidic to prevent their formation.
- Uric acid, calcium oxalate, and cystine form in acidic urine; keep the urine pH alkaline to prevent its formation.
What is the role of urine pH in drug therapy?
- Neomycin, kanamycin, and streptomycin are effective in alkaline urine.
- Salicylate intoxication keeps the urine pH alkaline.
- Sulfa therapy forms crystals, which can be prevented by keeping urine pH alkaline.
What is the role of urine pH in diseases?
- Keep urine pH acidic during the following:
- The treatment of urinary tract infection.
- In persistent bacteriuria.
- In renal calculi, which develop in alkaline urine.
What is the effect of diet on urine pH?
- Vegetarian diet, citrus fruits keep urine alkaline.
- A diet rich in protein (meat) keeps urine acidic.
- Cranberry juice keeps urine acidic, and some believe it is a remedy for UTIs.
How would you summarize the significance of the urine pH?
- For assessment of metabolic alkalosis and acidosis.
- For evaluation of respiratory alkalosis and acidosis.
- To find an unsatisfactory urine specimen.
- Urinary pH is important in the management of renal stones or crystals.
- Uric acid stones precipitate in acidic urine and are soluble in basic urine.
- Alkaline urine will precipitate calcium or calcium phosphate, and acidic urine will dissolve these stones.
- Role in the formation of renal calculi.
- Role of pH in the differentiation of the crystals in the urine.
- pH is important in the treatment of urinary tract infections.
- Alkaline urine is needed to treat sulfonamide and streptomycin therapy and prevent their precipitation in the kidneys.
- Alkaline pH is needed during blood transfusion and salicylate intoxication.
- In the case of cystitis, pH is kept acidic to fight bacterial infection and to prevent the formation of alkaline stones.
- Role of pH in renal tubular acidosis.
| Summary of urinary pH in various conditions |
|
What is the role of pH in various diseases?
| Characteristics | Etiology | Mechanism |
| Acidic urine | ||
|
|
|
|
|
|
|
|
|
| Alkaline urine | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
It is seen in:
|
|
|
|
|
What is the Normal picture of the urine?
| Physical features | Chemical features | Microscopic findings |
|
|
|
Questions and answers:
Question 1: What will be urine pH in respiratory or metabolic acidosis?
Question 2: What should be urine pH, in case of neomycin, kanamycin, and streptomycin therapy?