Urine Crystals (Crystalluria) and Their Significance
Urine Crystals (Crystalluria)
What sample is needed for urine crystals?
- Freshly voided urine is the best sample.
- Refrigerate, or if you keep it at room temperature, that will increase the formation of the crystals.
What are the precautions for urine crystals (Crystluria)?
- pH is very important to note.
- Taking the history of the medications will save time and energy.
- Refrigeration will precipitate out many crystals because of the change in the solubility of various crystals.
- Urine kept at room temperature leads to precipitation or dissolves the crystals.
- The radiographic dye can make crystals in dehydrated patients.
- Ampicillin and sulfonamides also give rise to crystal formation; this happens in dehydrated patients.
How will you define urine crystals (Crystalluria)?
- There may be well-defined crystals or amorphous material in the urine sediment.
- These appear as geometrical formed structures or amorphous materials.
- The presence of crystals in the urine is called Crystalluria.
- When urine is left at room temperature or refrigerated, it becomes cloudy because crystals or amorphous material precipitate.
What is the importance of the urine crystals (crystalluria)?
- These crystals are important in the case of kidney stones.
- Renal damage was caused by the crystals.
- In liver diseases.
- Inborn error of metabolism.
- Some of the crystals indicate some metabolic disorders like cystinuria or a sulfa drug.
- The stone formation may be without crystals in the urine, or crystalluria may be without stone formation.
- Crystals are seen mostly in concentrated urine.
- Crystals are divided into :
- Normal or abnormal.
- In alkaline or acidic urine.
- Crystals were found due to medication.
- Crystals found in acidic urine have a pH of <6.5; in alkaline urine, pH is >7.0.
How will you report crystalluria?
- Rare/HPF.
- FeW/HPF.
- Moderate/HPF.
- Many/HPF.
What are the factors influencing crystal formation?
- The kidneys are the main site for excreting the waste product of metabolism, which the body needs.
- Urea is derived from the metabolism of amino acids.
- Creatinine from the muscles.
- Uric acid from the nucleic acid.
- The hemoglobin end product is bilirubin.
- Hormones are excreted as hormone metabolites.
- Kidneys also excrete toxins and other foreign substances, such as pesticides, drugs, and food additives, produced by the body or ingested.
What is the mechanism of the formation of urine crystals?
- The crystalization of urine solutes forms crystals.
- The solutes are:
- Inorganic salts.
- Organic compounds.
- Medications by drug use.
- Precipitation is dependent upon:
- Temperature.
- pH.
- Solute concentration.
- As the concentration of solutes increases, their ability to remain in solution decreases, resulting in crystal formation.
- Solute precipitates more readily at low temperatures.
- So, if you keep the urine at room temperature or refrigerate it, then crystals are abundant.
- Organic and iatrogenic compounds crystalize more quickly in the acid urine.
- Inorganic salts are less soluble in neutral or alkaline pH.
- The exception is calcium oxalate, which precipitates in both acidic and alkaline mediums.
- The Slower crystalization leads to larger crystal formation, but the basic structure remains unchanged.
- Reversal of the pH will dissolve the crystals.
- Amorphous urates crystals formed in the refrigerated sample; if you warm the urine, these will disappear.
- Amorphous phosphate crystals need acetic acid to dissolve. But practically, this is not done because the acetic acid will distort the RBCs.
What are the factors that help in reporting the crystals?
- Always note the pH of the urine, which will help to identify the crystals.
- All abnormal crystals are found in acidic urine.
- Polarized microscopy also helps to identify the crystals.
What are the characteristics of crystals?
- Most of the urate crystals are yellow to reddish-brown.
- Amorphous urates appear in yellow-brown granules. These are in clumps and look like the granular cast.
- If urine is refrigerated, the amorphous crystals precipitate and give pink sediment.
- Amorphous urine crystals appear in the urine with a low pH of >5.5, and uric acid crystals appear when the pH is lower.
What are the types of crystals in acid urine?
Acidic-urine crystals are:
- Uric acid.
- Amorphous urates.
- Sodium urates.
- Cystine (these are rarely found).
- Cholesterol crystals (these are rarely found).
- Tyrosine (these are rarely found).
- Leucine (these are rarely found).
- Bilirubin.
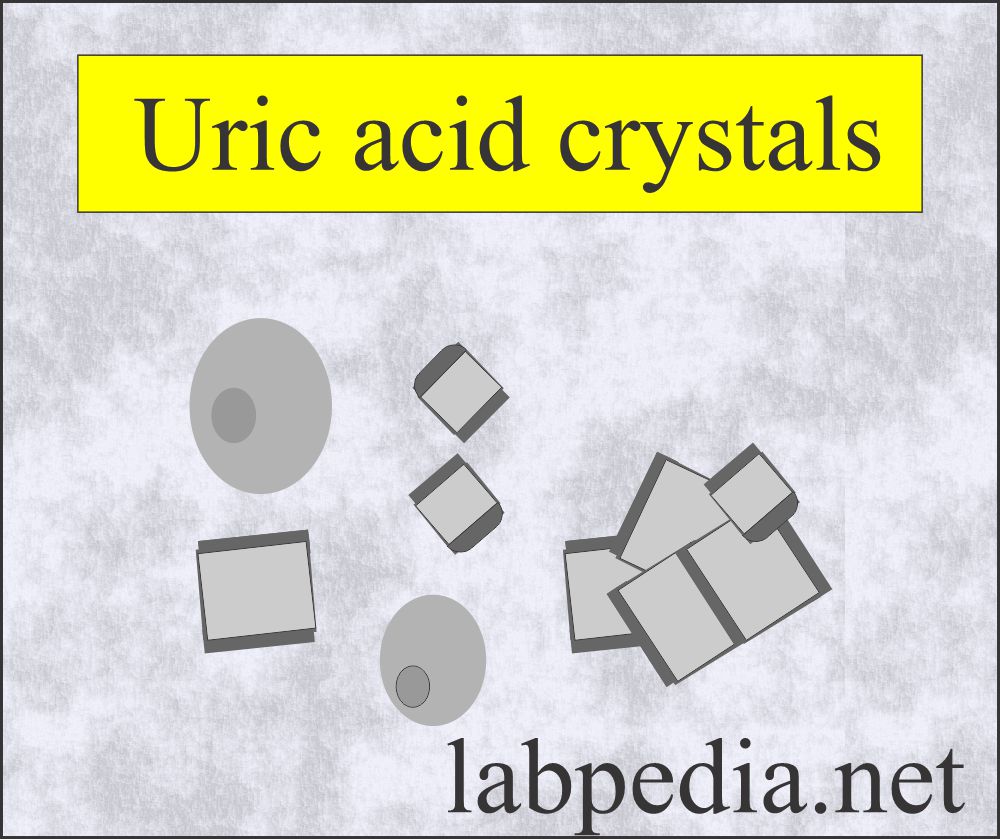
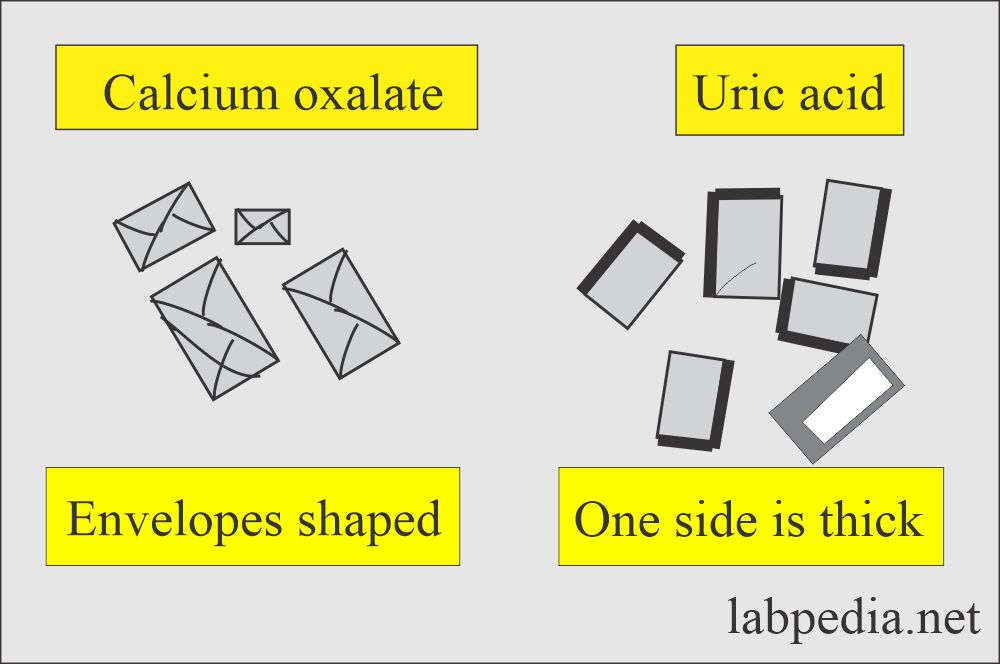
Uric acid crystals:
What are the characteristics of Uric acid crystals?
- Serum uric acid is raised in 40% to 50% of the patients.
- These are seen in various shapes, such as four-sided flat plates, wedge-shaped plates, and rosettes.
- These are usually yellow-brown.
- But maybe colorless and six-sided shapes like cystine crystals.
- These are birefringent in polarized light.
- These are common in patients with leukemia getting chemotherapy.
- Sometimes, these are seen in gout.
Acid urates and sodium urates:
- These are like amorphous urates and are seen in less acidic urine.
- These are seen along with amorphous urates and have little clinical significance.
- Sodium urate crystals are needle-shaped and appear in the synovial fluid during gout attacks and also in the urine.
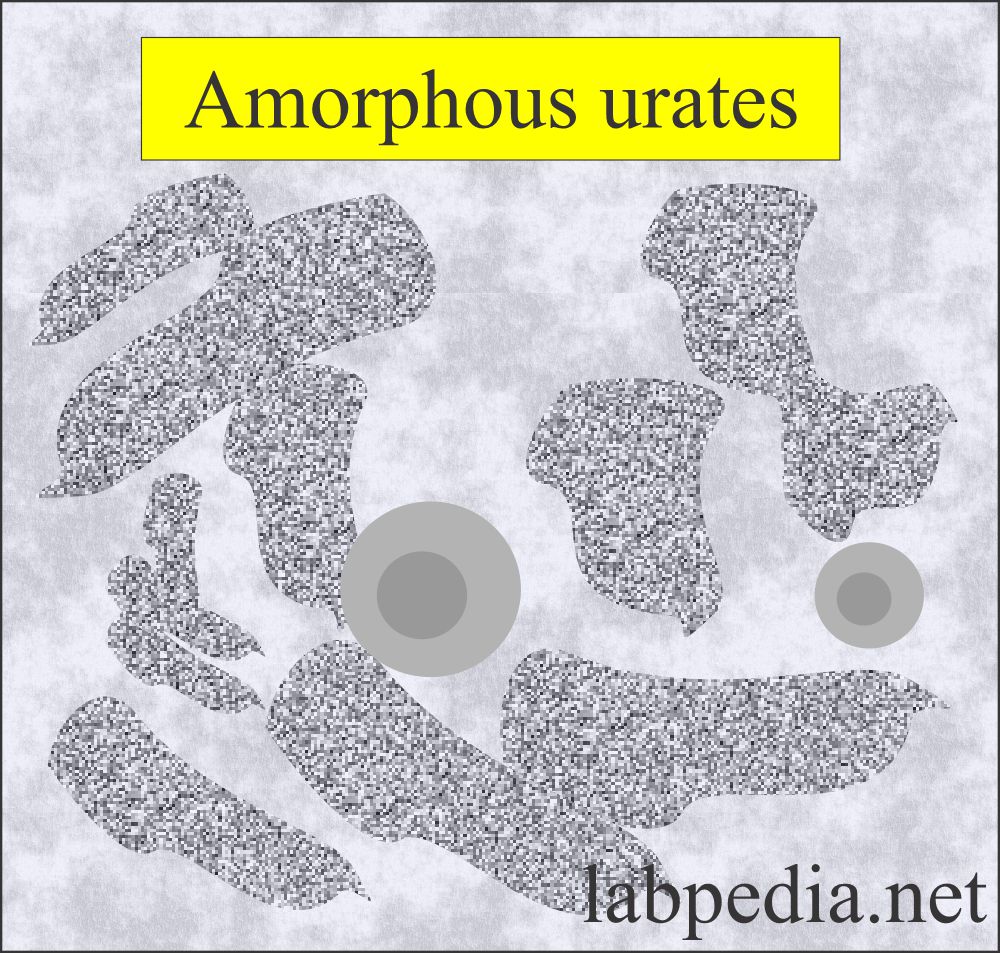
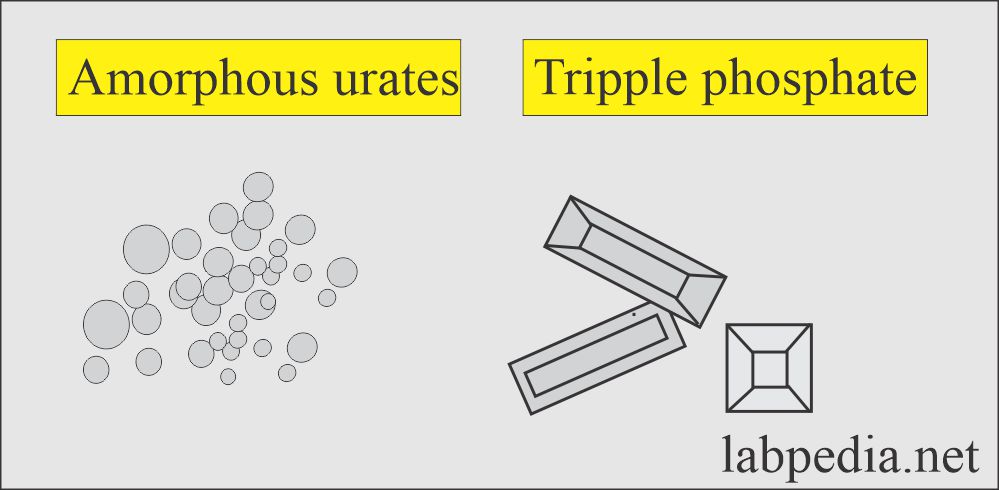
Amorphous urates:
What are the characteristics of amorphous urates crystals in urine?
- These are yellow-brown granules.
- These may appear in clumps resembling granular casts.
- These are brick dust or yellow-brown.
- These crystals are found in acidic urine with a pH >5.5 (acidic urine).
- These are soluble in an alkaline medium.
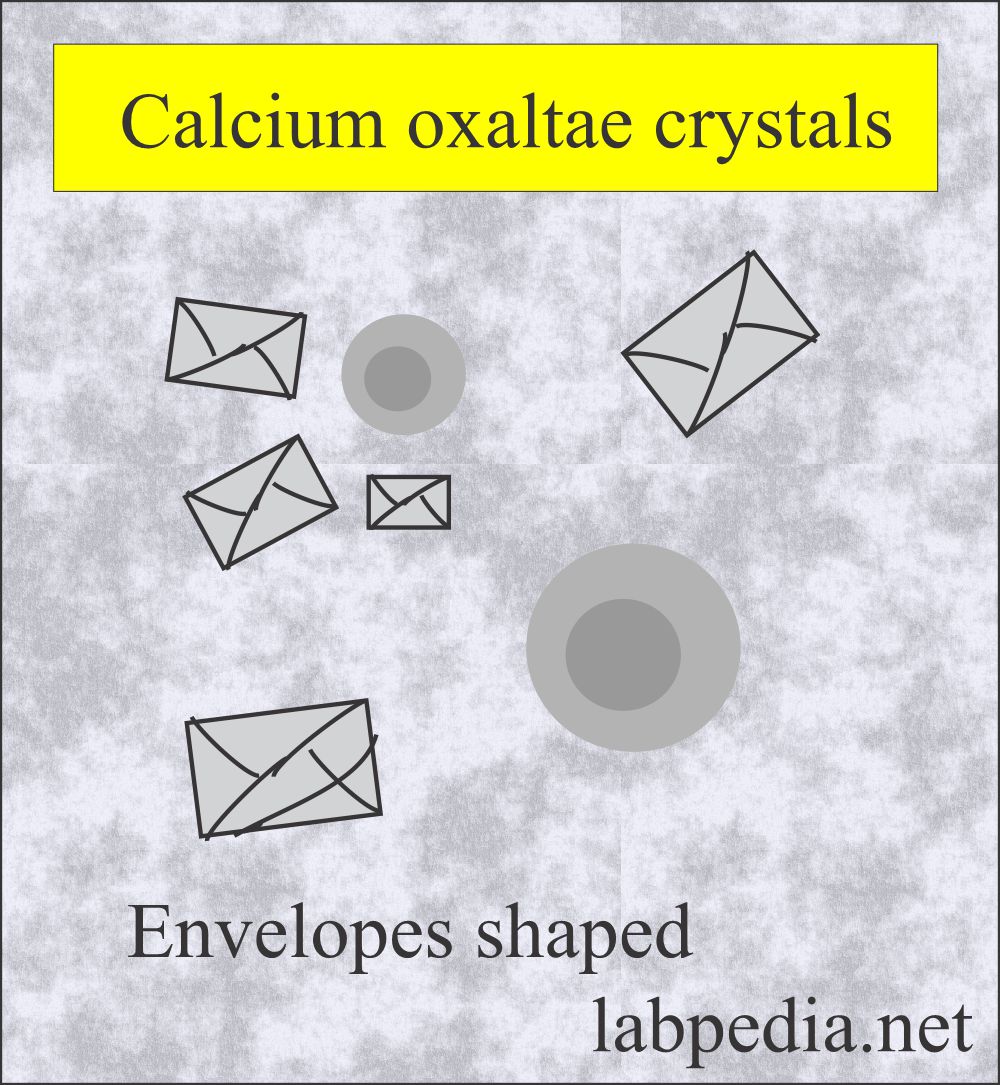
Calcium oxalate:
What are the characteristics of Calcium oxalate crystals in urine?
- More common in acidic urine but may be seen in neutral or alkaline urine.
- The most common is dihydrate, a colorless, octahedral envelope shape, or two pyramids joined at their bases.
- Monohydrate crystals are oval or dumble-shaped.
- In polarized light, both are birefringent.
- The finding of clumps of calcium oxalate crystals indicates renal stone formation.
- These are commonly seen when taking foods like tomato, asparagus, and ascorbic acid.
- Monohydrate crystals are seen in ethylene glycol (antifreeze) poisoning.
- These are soluble in dilute hydrochloric acid.
What are the Crystals in the acidic urine?
| Name of crystals | pH | Effect of heating (solubility) | Shape | Color |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Urine Crystals (Crystalluria): Calcium oxalate and uric acid crystals
What are the types of crystals in alkaline urine?
Alkaline urine crystals are:
- Calcium phosphate.
- Amorphous phosphates.
- Calcium carbonate.
- Ammonium biurate.
- Tripple phosphate.
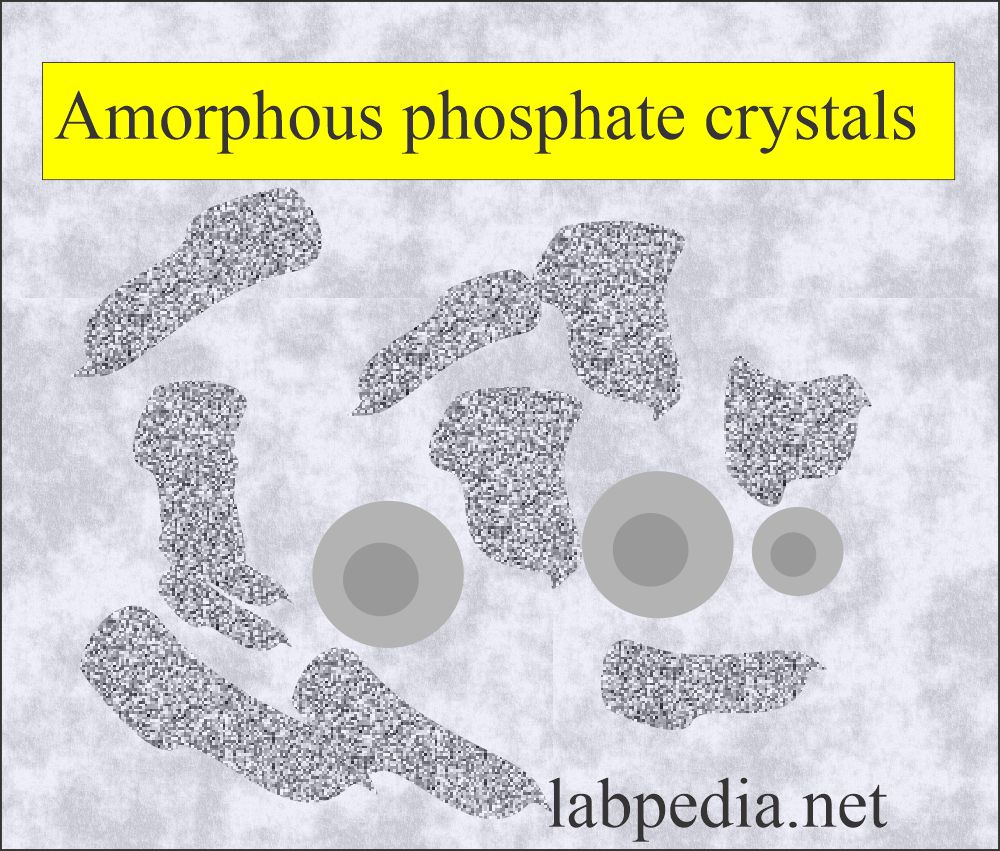
Amorphous phosphate:
What are the characteristics of amorphous phosphate?
- These are granular in shape, like amorphous urates.
- If refrigerated, these produce a white precipitate, which does not dissolve when warming.
- These are differentiated from the urates by color and the urine pH.
- These are soluble in an alkaline or neutral medium.
- These are soluble in dilute acetic acid.
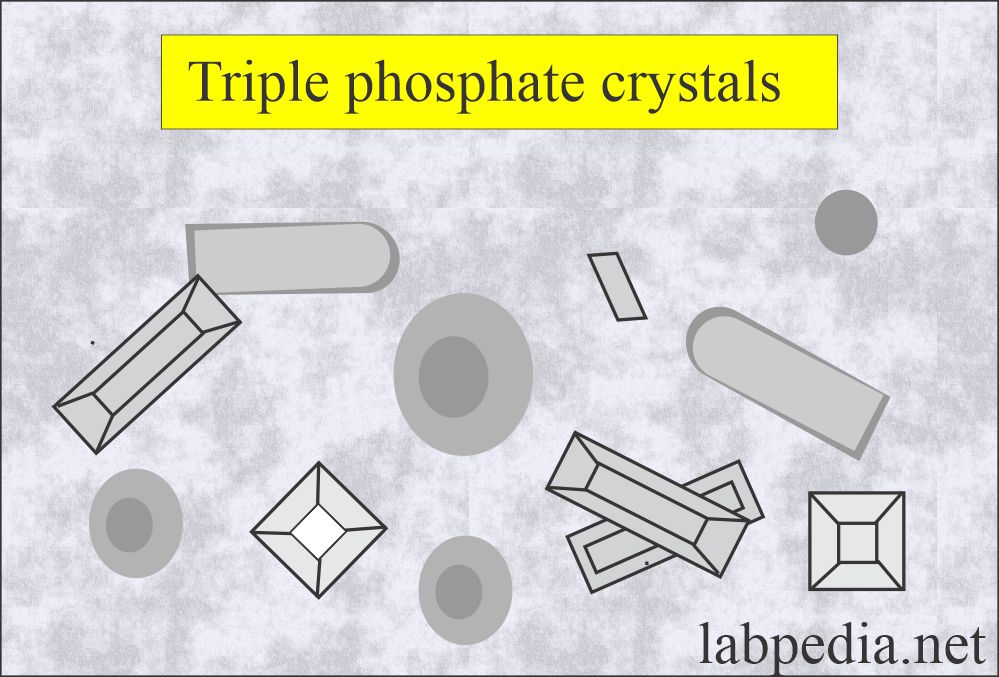
Triple phosphate (Ammonium magnesium phosphate):
What are the characteristics of triple phosphate crystals?
- These are seen in alkaline urine.
- These are colorless, prism-shaped, resembling the coffin lid.
- Under polarized lights are birefringent.
- These have no clinical significance.
- These are seen in an alkaline medium.
- These are soluble in dilute acetic acid.
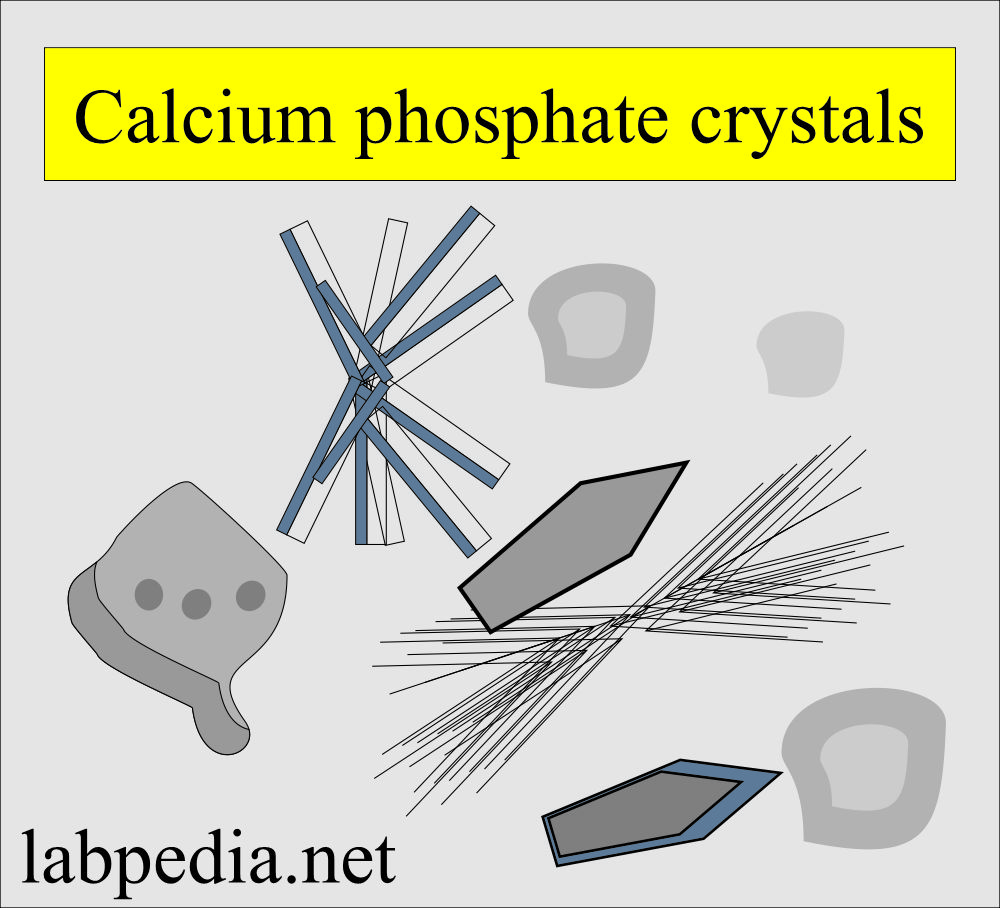
Calcium phosphate:
What are the characteristics of calcium phosphate crystals?
- These are colorless, flat, rectangular plates.
- Or thin prism, often in rosette forms.
- Rosette forms need to be differentiated from the sulphonamide crystals.
- These crystals dissolve in dilute acetic acid, while sulphonamide crystals will not.
- These have no clinical significance.
- These are seen in alkaline or neutral pH.
- These are soluble in dilute acetic acid.
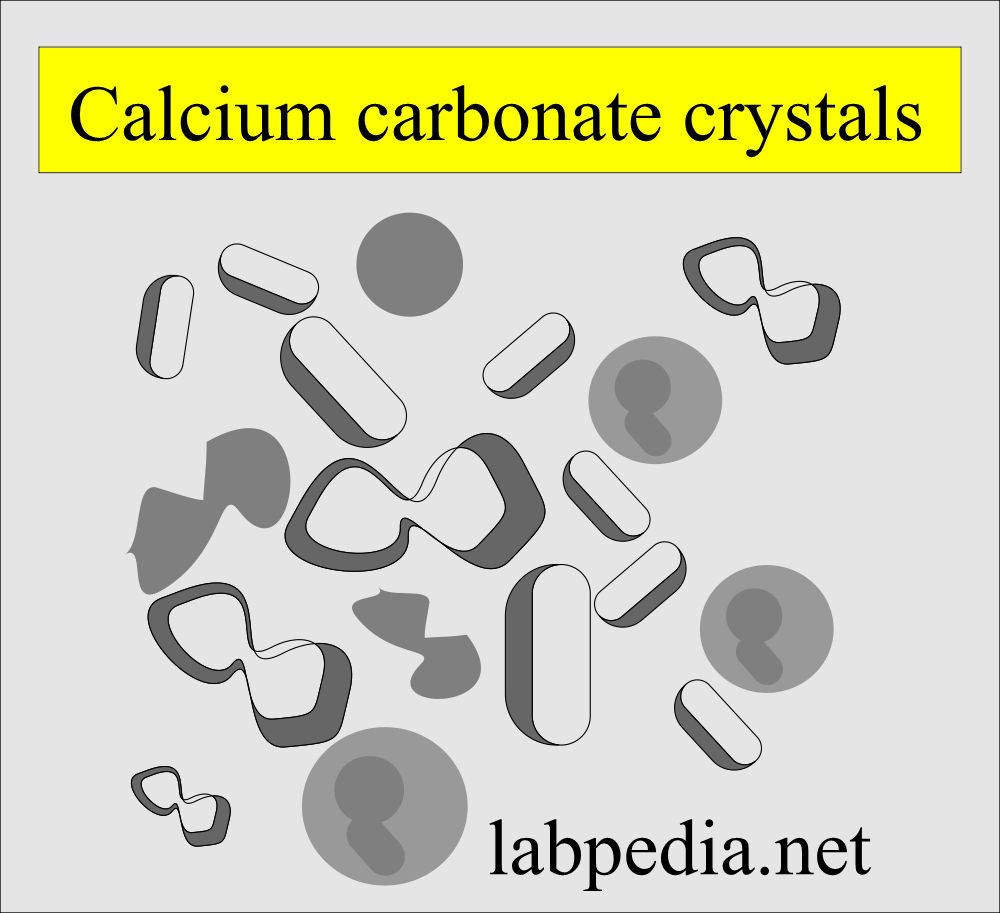
Calcium carbonate:
What are the characteristics of calcium carbonate crystals?
- These are small. Colorless, with a dumbbell or spherical shapes.
- These may occur in clumps and resemble amorphous material.
- If you add acetic acid, then gas formation occurs.
- They are birefringent in polarized light, which differentiates them from the bacteria.
- This crystal has no clinical significance.
- These crystals are seen in an alkaline medium.
- These are soluble in acetic acid with gas formation.
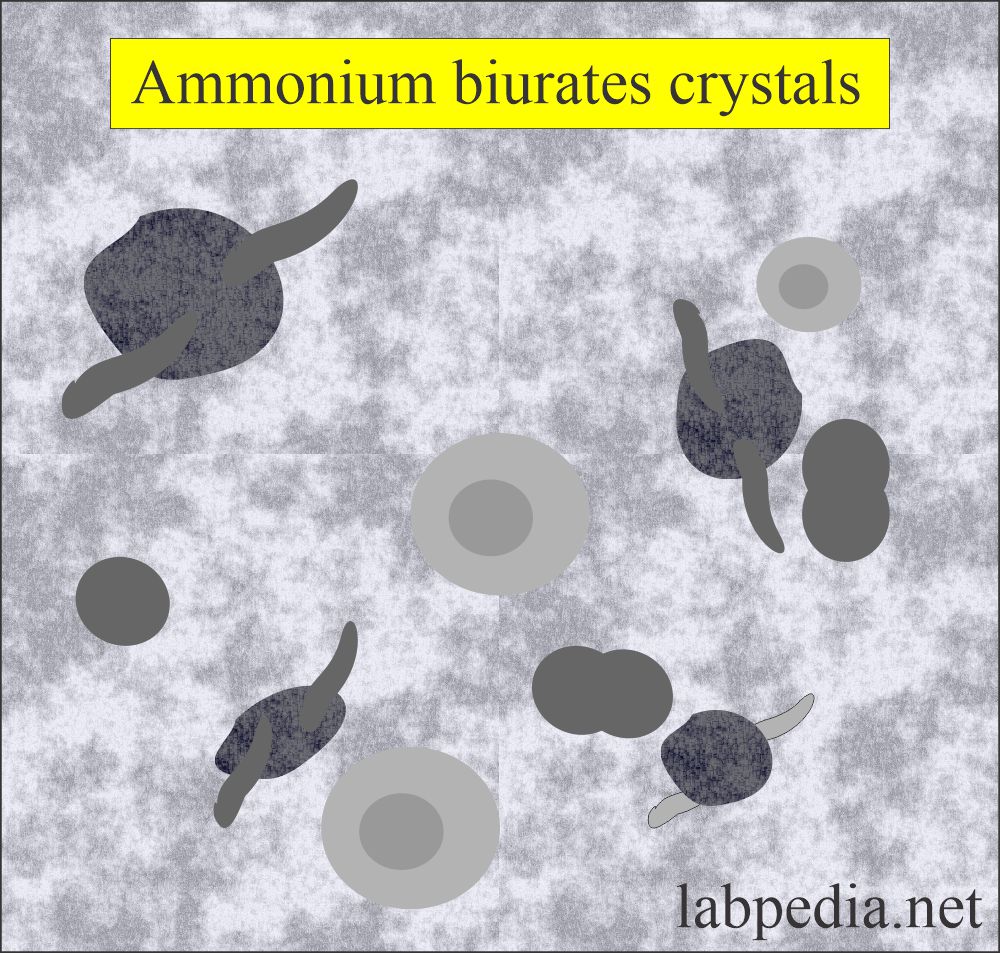
Ammonium biurates:
What are the characteristics of ammonium biurates crystals?
- These have characteristic yellow-brown colors.
- These are usually described as thorny apples because of the spicule-covered spheres.
- These dissolve at 60°C.
- If you add glacial acetic acid, it will change into uric acid.
What are the Crystals in alkaline urine?
| Name of crystals | pH of urine | Effect of heat | Color and shape |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What is the solubility of various crystals?
| Crystals | Color | pH | Soluble in |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What are the Abnormal crystals in urine?
- Metabolic origin:
- Tyrosine.
- Cystine.
- Cholesterol.
- Leucine.
- Bilirubin.
- Hemosiderin.
- Drugs origin:
- Sulfonamides.
- Radiographic contrast media.
- Ampicillin.
Cystine crystals:
What are the characteristics of cystine crystals in urine?
- These are seen in the inborn error metabolic disorder when the cystine is not absorbed by the renal tubules (cystinuria).
- There is a tendency to form renal calculi.
- These are colorless, hexagonal plates and may be thick or thin.
- In the presence of ammonia, these crystals disintegrate.
- Cysteine stones are seen in 1% to 2% of the cases.
- The cyanide-nitroprusside test is needed to confirm the cystine crystals.
- These crystals appear in an acidic medium.
- It is soluble in ammonia and dilutes HCL.
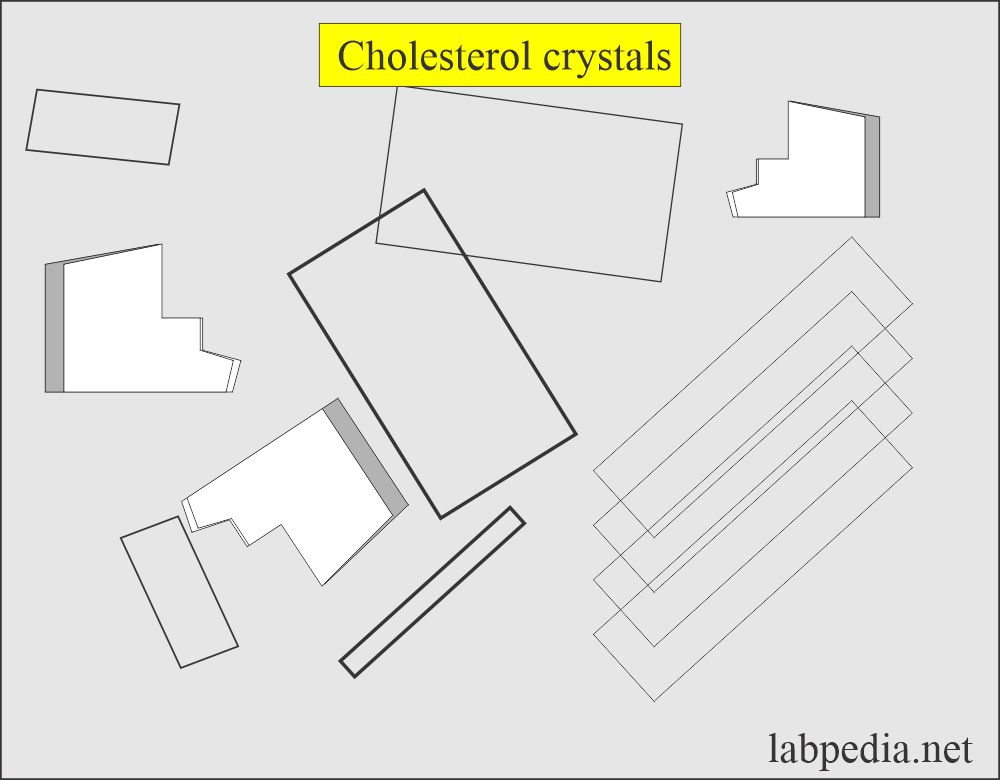
Cholesterol crystals:
What are the characteristics of cholesterol crystals in urine?
- These are not seen unless the urine is refrigerated.
- These have a characteristic appearance resembling rectangular plates with a notch in one or more corners.
- These are seen as disorders producing lipiduria in nephrotic syndrome, along with fatty acids and fat oval bodies.
- In polarized light, these are birefringent.
- This crystal appears in an acidic medium.
- These are soluble in chloroform medium.
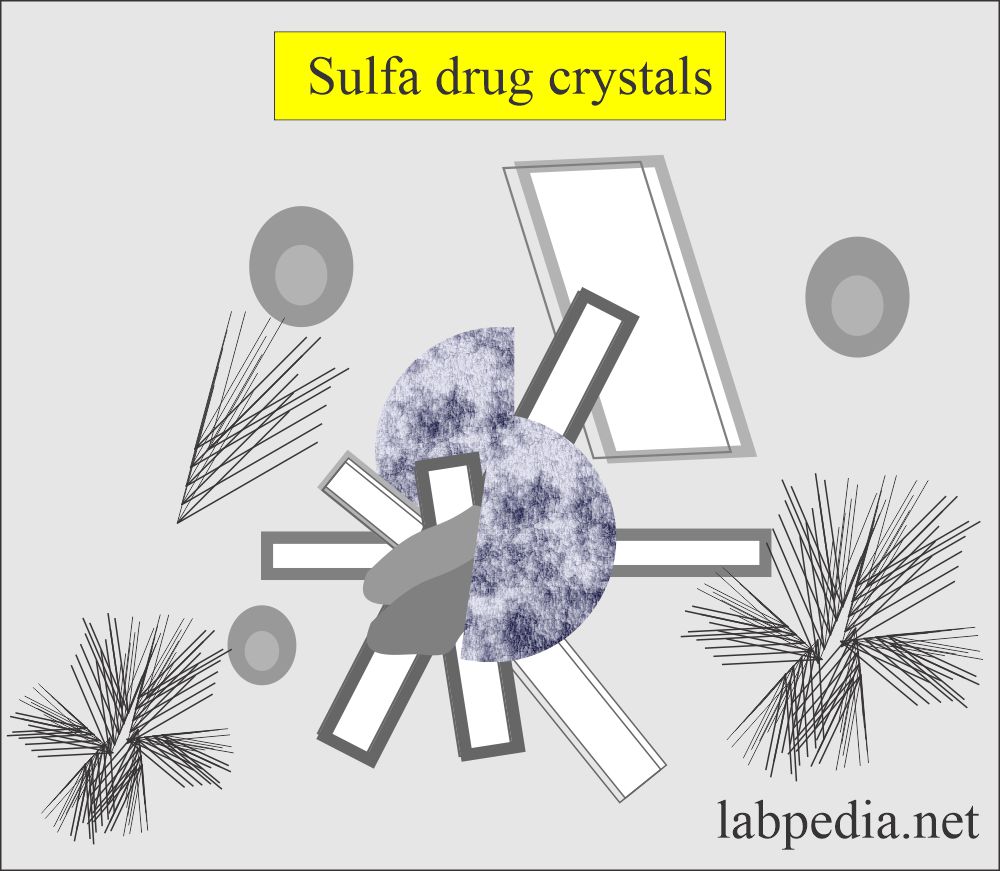
Sulfonamide crystals:
What are the characteristics of sulfonamide crystals in the urine?
- There are a variety of crystal shapes and colors.
- Shapes variables like needles, whetstone, rhombic, wheat, and rosette with color ranging colorless to yellow-brown,
- The patient’s history will help you diagnose these crystals.
- The diazo reaction can confirm these crystals.
- These crystals are seen in an acid/neutral medium.
- These crystals are soluble in acetone.
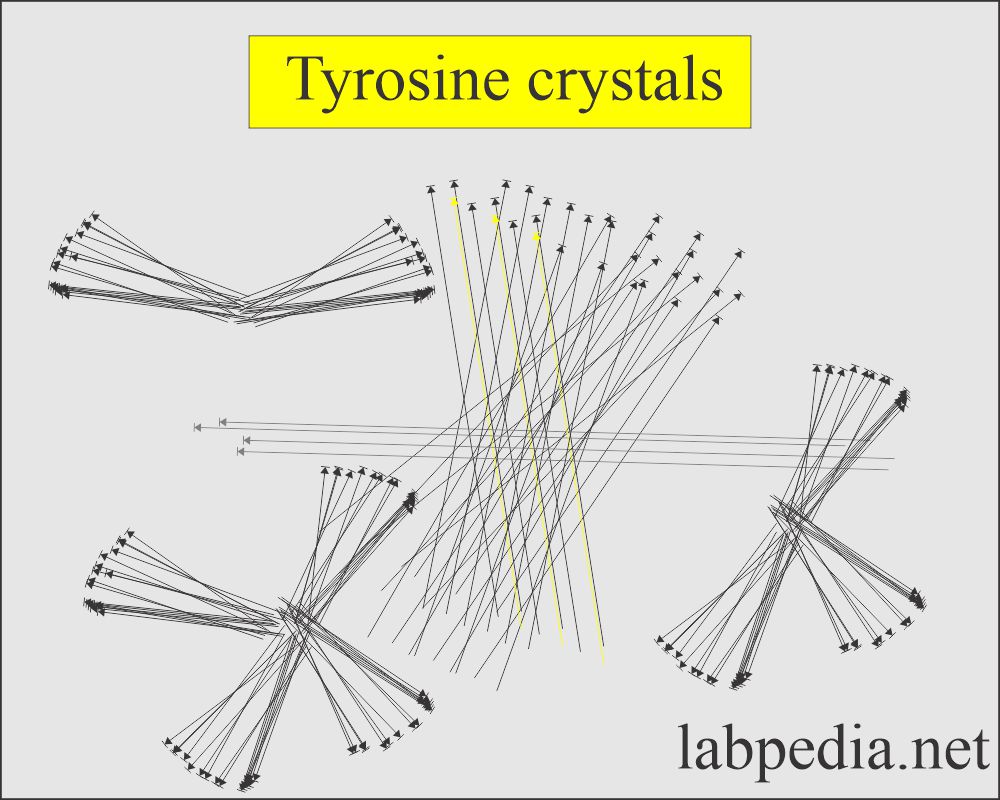
Tyrosine crystal:
What are the characteristics of tyrosine crystals in the urine?
- These are fine colorless to the yellow needle-like structures that form clumps or rosettes.
- These may be seen in inherited disorders of amino acid metabolism.
- These crystals are formed in an acid/neutral medium.
- These crystals are soluble in alkali or by heat.
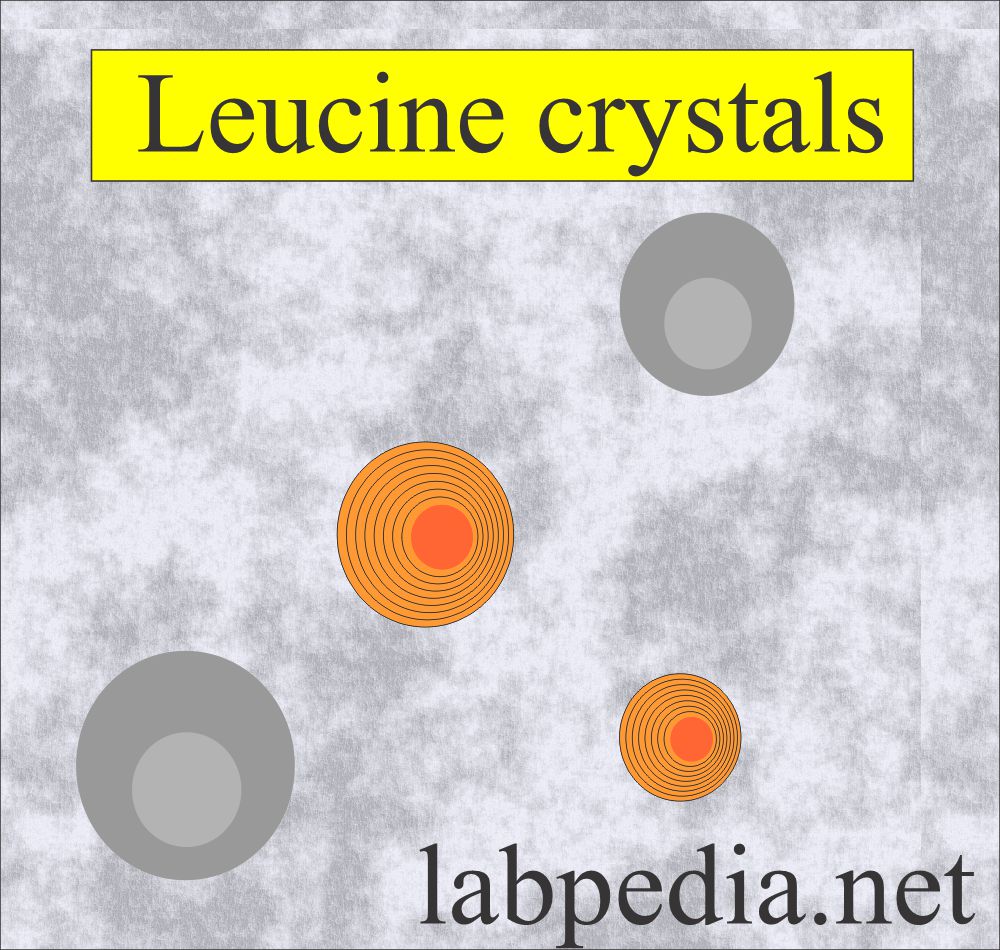
Leucine crystals:
What are the characteristics of leucine crystals in the urine?
- These are seen because of the defect in the amino acid leucine.
- These are yellow-brown spheres that will show concentric circles and radial striations.
- These are also called wagon wheels.
- These crystals form in an acid/neutral medium.
- These are soluble in hot alcohol and alkali.
- These are less frequent than tyrosine crystals.
- These are accompanied by tyrosine crystals.
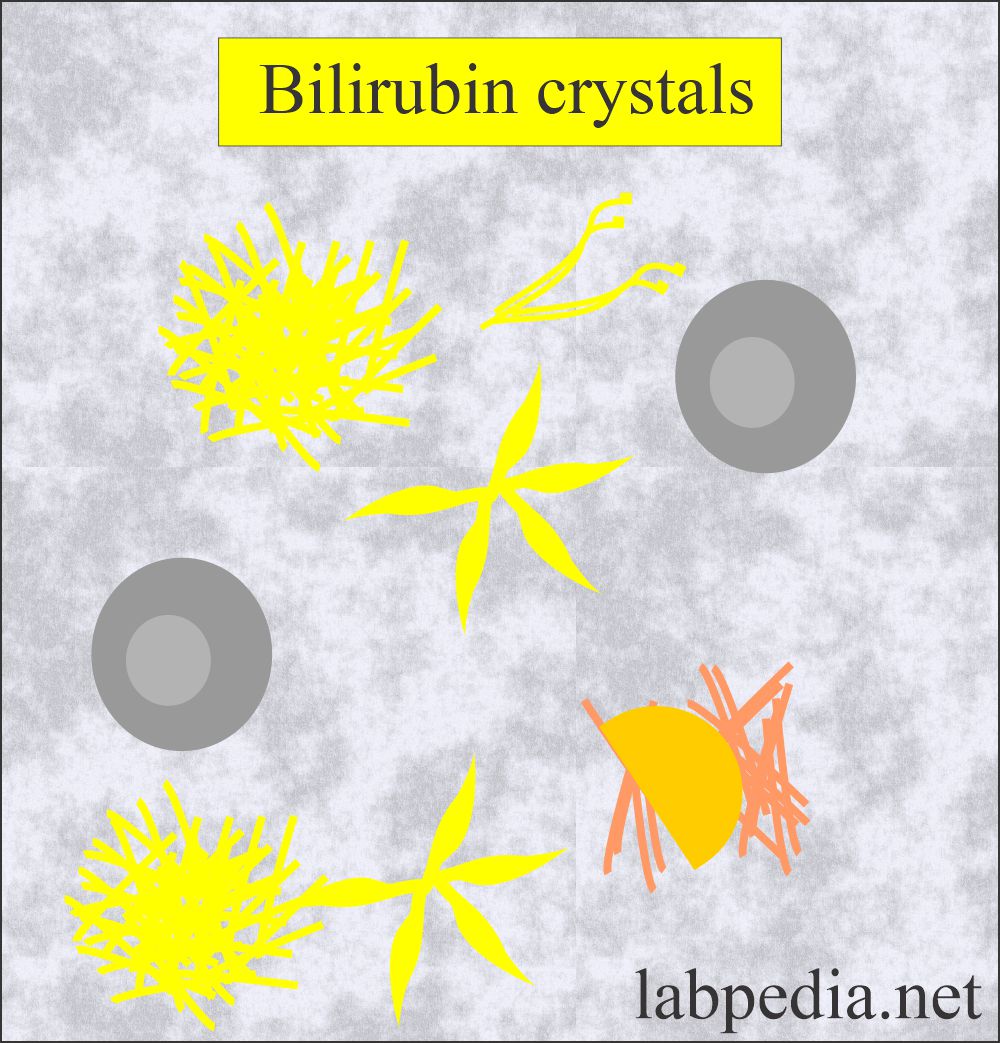
Bilirubin Crystals:
What are the characteristics of bilirubin crystals in the urine?
- Bilirubin crystals are present in liver diseases where an increased amount of bilirubin is excreted in the urine.
- These crystals are clumped needles or granules, and the characteristic color of the bilirubin crystals is yellow.
- The chemical reaction for the bilirubin is positive.
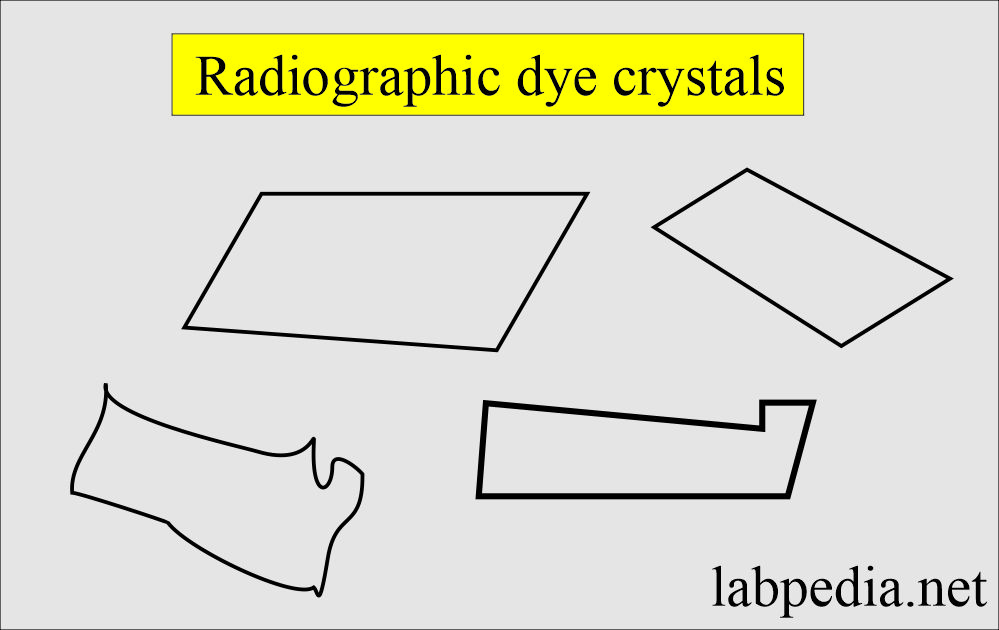
Radiographic material crystals:
What are the characteristics of radiographic material crystals in the urine?
- These crystals are colorless.
- These crystals appear in an acid medium.
- These crystals are soluble in 10% NaOH.
What is the significance of crystals in the urine?
- Calcium oxalate crystals in clumps indicate renal stone formation.
- Calcium oxalate crystals are abundant in foods with high oxalic acids, such as tomatoes, asparagus, and ascorbic acid.
- Monohydrate oxalate crystals are seen in ethylene glycol poisoning (antifreeze material).
What are the possible contaminants and artifacts?
There are a few contaminants that interfere with the microscopy of the sediments:
- Starch.
- Fibers, including diaper fibers.
- Oil droplets.
- Air bubbles.
- Pollin grains.
- Fecal contamination.
- Glass fragments.
Questions and answers:
Question 1: What are the most common crystals and renal calculi.
Question :
Question 2: What are the artifacts in the urine examination?
Question 3: What will be the pH for the bilirubin crystals??