Tumor Markers:- Part 11 – Carcinoembryonic Antigen (CEA)
Carcinoembryonic Antigen (CEA)
What sample is needed for Carcinoembryonic Antigen (CEA)?
- This test is done on the patient’s serum.
- The serum is stable for 24 hours at 2 to 8 °C.
- For a long time, freeze the serum at -20 °C.
- Some methods may use plasma.
- For plasma use EDTA 2mg/mL.
- No special preparation is needed.
- This test can be done on peritoneal fluid, and CSF, if it is raised, will indicate tumor metastasis.
What are the precautions for Carcinoembryonic Antigen (CEA)?
- Smokers have a higher CEA level.
- Keep in mind that benign conditions like colitis, diverticulitis, and cholecystitis give raised CEA levels.
- Also, liver diseases give to raise the CEA level.
What are the Indications for Carcinoembryonic Antigen (CEA)?
- CEA is a tumor marker for:
- Colorectal carcinoma.
- Gastrointestinal carcinoma.
- Lungs malignancies.
- Breast cancers.
- This tumor marker is used to find the extent of disease in patients, particularly gastrointestinal cancers.
- Determination of the prognosis of colon cancer.
- This may be used in breast cancers.
- This test is used to monitor the disease and treatment.
How will you define Carcinoembryonic Antigen (CEA)?
- History of CEA:
- CEA was discovered in 1965 by Gold and Freeman.
- This experiment was done by immunizing rabbits with human colon cancer cell extract.
- This was found in the serum of a patient with colorectal carcinoma.
- The antigen, which was found in embryonic tissue, was named carcinoembryonic antigen.
- Normal function of CEA:
- In adults, there is a minimal function, and present in very low amounts.
- In the fetus, CEA functions as a cell adhesion molecule.
- Thus, this CEA antigen was considered the indicator of colorectal cancers.
- Later on, it was found in other various tumors like breast, stomach, pancreas, hepatobiliary tumors, and sarcomas.
- This was also found in benign conditions like ulcerative colitis, cirrhosis, and diverticulitis.
- Chronic smokers also have raised the level of CEA.
- Carcinoembryonic Antigen (CEA) indicates the bulk of the tumor.
What is the structure of the Carcinoembryonic Antigen (CEA)?
- The tissue found in embryonic tissue is called carcinoembryonic antigen (CEA).
- CEA is normally found in the fetal gut tissue.
- By the time the detectable birth level of CEA disappears.
- There are about 10 genes located on chromosome 19 that encode the CEA protein.
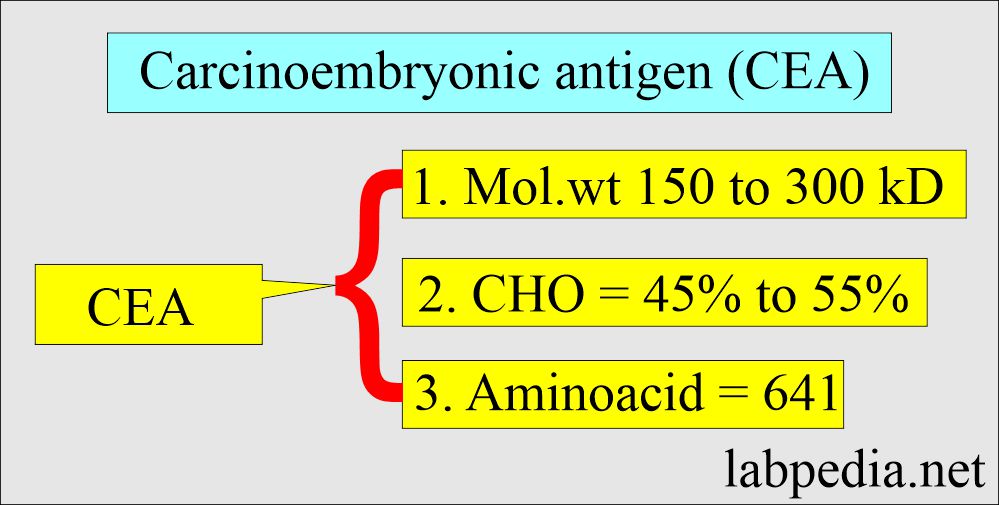
- This is a glycoprotein with a molecular weight of 150 to 300 kD.
- It contains 45% to 55% carbohydrates.
- It is a single polypeptide chain consisting of 641 amino acids, with lysine at its N-terminal.
- CEA proteins are encoded by 10 genes located on chromosome 19.
- CEA is a part of the immunoglobulin gene ” superfamily.”
- CEA is a tumor-associated, oncofetal antigen seen in embryonic and fetal tissue.
- CEA is a glycoprotein that normally occurs in fetal gut tissue.
How will you interpret Carcinoembryonic Antigen (CEA)?
- CEA is increased in:
- Colorectal cancer = 70%.
- Gastric = 50%.
- Pancreatic = 55%.
- Lung = 45%.
- Breast = 40%.
- Ovarian = 25%.
- Uterine = 40%
- CEA is not used to screen for cancers.
- This is more specific for colorectal carcinoma.
- In a patient with colorectal carcinoma, its level correlates with the tumor stage, tumor grade, and tumor site.
- Well-differentiated tumors produce more CEA than poorly differentiated tumors.
- CEA is less commonly increased in lymphoma, leukemia, and malignant melanoma.
- CEA has also been seen in an HIV-positive patient with P.C.carinii, a heavy smoker, and inflammatory bowel disease.
- This is metabolized in the liver, so in liver diseases, it is increased.
- Its median value is higher for smokers than non-smokers.
- It is useful for monitoring GIT cancer, especially colorectal carcinoma.
- CEA is increased by 60% to 90% of metastasis of lung cancer.
- If the CEA level increases from the baseline after surgery, it indicates the tumor’s recurrence.
- Patients with high preoperative concentration have a poorer prognosis than those with low values.
What is the role of Carcinoembryonic Antigen (CEA) in colon cancer?
- After the surgery and removal of colon cancer, CEA takes 6 to 12 weeks to become normal.
- Failure to become normal suggests incomplete surgery for colon cancer.
- A progressive increase in the CEA indicates the recurrence of colon cancer.
- Monitor the patient by estimating CEA every 2 to 3 months in stage II and stage III colon cancer cases for 2 or more years.
- What is the effect of CEA on staging?
- Increased concentration of CEA (>3 ng/mL) indicates a poor prognosis in that stage of the tumor-like:
- In Dukes, stage A = 28%.
- Dukes stage B = 45%
- Dukes stage C = 70%
- Before therapy, CEA <5 ng/mL suggests localized disease and a favorable prognosis.
- CEA >10 ng/mL suggests advanced disease and poor prognosis.
- If CEA is >20 ng/mL, 80% of patients have a recurrence within 14 months after surgery.
- The pattern of CEA changes during chemotherapy.
- CEA response to therapy:
- If there is no decrease in the CEA level with therapy, indicating the unresponsiveness of the tumor.
- In case of a decrease, indicate a responsive tumor.
- If there is a surge of CEA for weeks followed by a decrease in the level, it indicates a responsive tumor.
- An immediate decrease in CEA followed by an increase indicates an unresponsive tumor.
What are the drawbacks of Carcinoembryonic Antigen (CEA)?
- CEA can be detected in benign and malignant conditions.
- CEA is not produced in undifferentiated cancer.
- 30% of metastatic colon cancer has no increased CEA level.
- CEA is not raised in all colorectal cancers. Therefore, it is not a reliable screening test.
- Its use is limited to knowing the prognosis and monitoring the tumor response to antineoplastic therapy in patients with cancer.
- CEA is helpful in patients with breast and gastrointestinal malignancies.
What is the normal Carcinoembryonic Antigen (CEA)?
Source 1
Nonsmoker
- 99% = <5 ng/mL
- 1% = 5.1 to 10 ng/mL
- 0% = >10 ng/mL
Smoker
- 95% = <5.0 ng/mL
- 4% = 5.1 to 10 ng/mL
- 1% = >10 ng/mL
Source 2
- Adult (Non Smoker) = < 2.5 ng/ml
- Adult (Smoker) = < 5 ng/ml
What is the use of CEA testing?
- Diagnosis:
- It can be used as a diagnostic test along with other workups of the patients.
- Monitoring of the tumor:
- It is used to monitor the treatment response.
- It can detect recurrence after the treatment.
- Prognosis:
- A raised level of CEA indicates an advanced disease or poor prognosis.
- CEA is also useful for monitoring breast, lung, gastric and pancreatic carcinoma.
- Early diagnosis of breast cancer does not elevate CEA.
What are the causes of raised Carcinoembryonic Antigen (CEA) in benign conditions?
- In cirrhosis (45%).
- Pulmonary emphysema (30%).
- Benign breast disease (15%).
- Ulcerative colitis (15%).
- Rectal polyp (5%).
- Pancreatitis.
- Smoking.
- Inflammation.
- Hypothyroidism.
- Inflammatory bowel disease.
- Peptic ulcer.
What are the causes of raised Carcinoembryonic Antigen (CEA) in malignant conditions?
- Colorectal cancer (70%).
- In Duke’s stage A = 28%.
- In Duke’s stage B = 45%.
- Lung (45%).
- Breast cancer (40%).
- Gastric carcinoma (50%).
- Giant cell carcinoma of the thyroid.
- Pancreatic cancer (55%).
- Ovarian cancers (25%).
- Uterine carcinoma (40%).
What are the important facts about Carcinoembryonic Antigen (CEA) in colon cancer?
- CEA level varies inversely with the grade, and well-differentiated tumors produce more CEA than poorly differentiated carcinoma.
- This is not a reliable screening test for cancer. This is good for monitoring the recurrence of colon cancer.
- Pretreatment CEA level is a good indicator of tumor burden and prognosis.
- The preoperative level of >5 ng/mL is a poor prognostic indicator.
- After surgery, it should come to a normal level.
- Persistently raised levels after surgery indicate the presence of the disease and need further workup.
- Patient with a lower or normal level has a lower disease than a high level, indicating advanced disease and maybe metastasis.
- A drastically low level after surgery indicates an almost complete cure for the tumor.
- If raised levels are 5 times the normal, then laparotomy is positive in 90% of the cases.
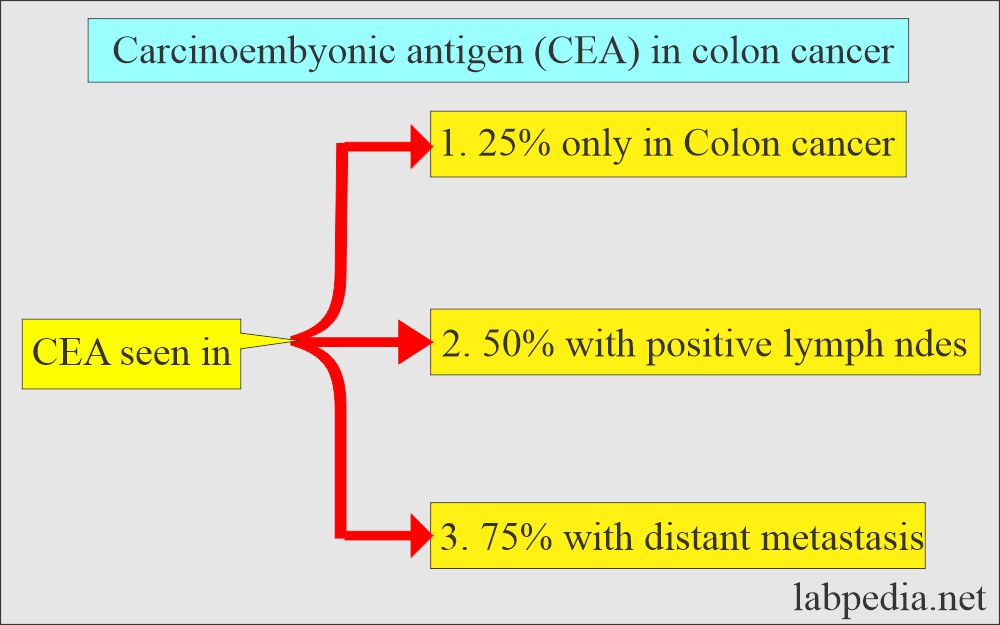
- CEA is raised in only 25% of cancer confined to the colon, 50% positive nodes, and 75% with distant metastasis.
- Left-sided cancers have more raised CEA than right-sided tumors.
- Bowel obstruction produces a higher level of CEA.
- In the case of liver diseases, its level is raised because it’s metabolized in the liver.
- A patient with a high preoperative CEA level has a worse outcome than a low level.
- Note: Our findings are from different sources.
Questions and answers:
Question 1: Can we see the raised level of CEA in benign conditions?
Question 2: Where is the site of CEA in the fetus?