Solutions:- Part 3 – Solutions with various examples
Solutions with various examples
- Here are some examples of various solutions commonly used in clinical laboratories.
0.1 M Hydrochloric acid solution
- This is 0.1 moles of HCl in one liter of solution.
- It is 3.65 g/1000 mL.
1 M solution of H2SO4
- This 1 molar solution = 98.08 g H2SO4 / L of the solution.
1 mol HCl/L
- Take 88 ml of concentrated HCl and add a solution to one liter with water.
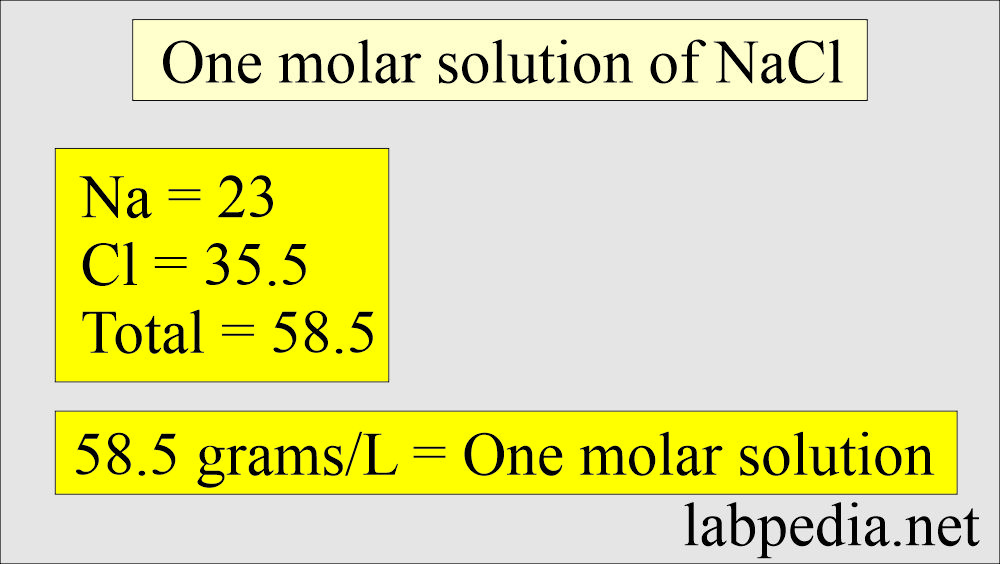
1 M solution of sodium chloride NaCl
- The gram-molecular weight is derived by finding the sum of the atomic weight:
- Na = 23
- Cl = 35.5
- Gram-molecular weight = 58.5
- 58.5 g of NaCl is dissolved in one liter (58.5 g/L).
10% solution of Na2SO4
- This is a weight/volume solution.
- 10 g/100 mL