Solutions:- Part 1 – Solutions Preparation used in Clinical Laboratory, and Dilution Formulas
Solutions Preparation
What is the definition of the solution?
- A solution is a homogenous mixture of one or more substances called solutes dispersed molecularly in a sufficient quantity of dissolving medium called solvent.
- When a solution holds as much dissolved solute as it can at a specific temperature, it is called a saturated solution.
- The solution may be gaseous, liquid, or sold.
What is the summary of the different solutions?
- Mole = Mass in grams (g)/gram molecular weight (g).
- The molarity of a solution = Numbers of moles of solute/Number of liters of solution.
- The molality of a solution = Number of moles of solutes Number of kilograms of solvent.
- Normality of a solution = Number of gram equivalents of solute/Number of liters of solution.
- Gram equivalent weight = Weight of the formula of substance/ Difference in oxidation state
What are the factors needed for Saturation of the solution?
- Temperature.
- Atmospheric pressure.
- Nature of the solute.
- Nature of the solvent.
What are the precaustions for the Preparation of solutions?
- Use the balance of good sensitivity.
- Use chemicals of analytical grade. Always use pure reagents.
- In the case of hygroscopic chemicals, weigh those rapidly.
- Use calibrated, clean glassware.
- Use carefully automatic pipettes.
- Clean the weighing containers, and if possible, clean them with the solvent.
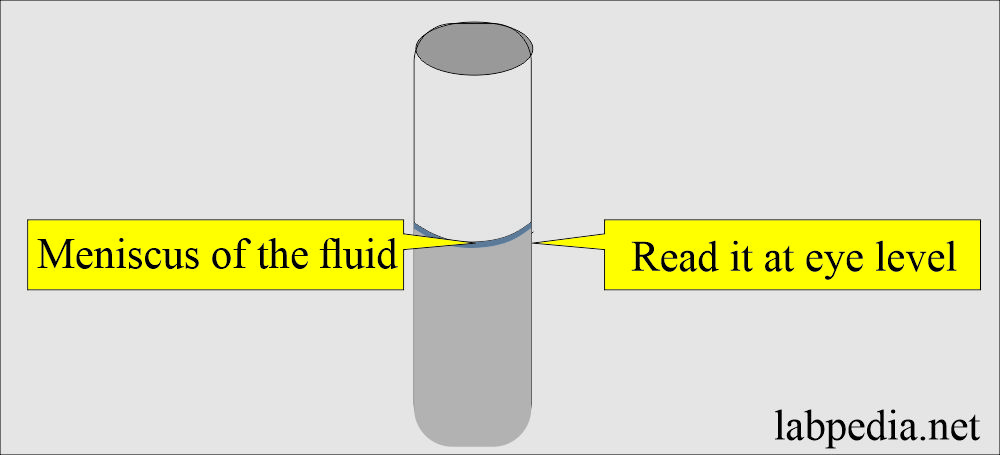
- Ensure the bottom of the fluid meniscus is on the graduation mark when viewed at eye level.
- Mix the solution properly; at least invert the flask 20 times.
- Store the solution in a clean and air-tight bottle.
- Store them in a colored bottle in case of the light-sensitive solution.
- Label the solution with clear print, and write the date of preparation. If possible, the expiry dates.
- Mostly solutions are stored at 2 to 8 °C.
- If the solution is harmful, label it as toxic.
- Protect all solutions from direct sunlight.
- Avoid small quantities; there are more chances for the mistake. Prepare a concentrated solution and use it with working dilution.
- Always use clean water; deionized water is best for preparing the solution.
What are the common solutions used in routine?
What is a Percent solution?
- This is defined as parts per 100, representing the percent (%).
- This is independent of the molecular weight of the substance.
- This is expressed as a solute concentration as a percent % (per hundred parts of the total solution).
- To make a 5% glucose solution, add 5 grams of glucose in 100 ml of distal water.
What are the Percent solution types?
What is Weight/weight (weight per unit weight)?
- The % of a solute or grams per 100 grams of the final solution.
- Both solutes and the solvent are weighed, and the total equals 100 g.
- Example:
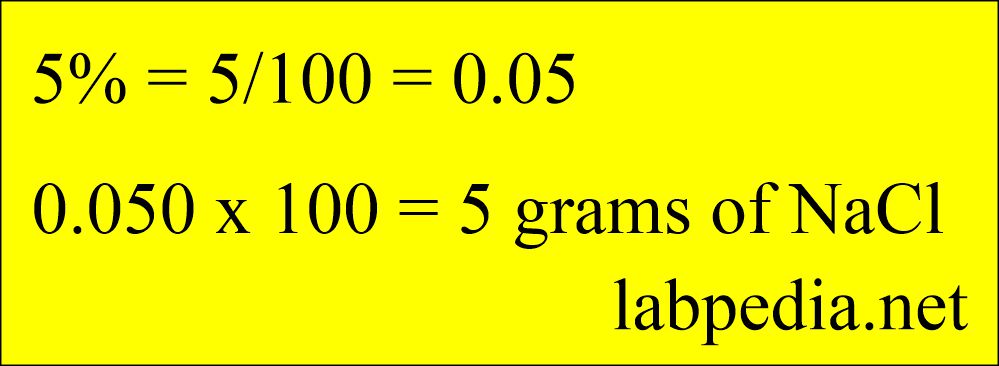
- To make 5% of an aqueous solution of NaCl containing 50 grams of NaCl and 950 g of diluent.
- Calculation =
- 5% = 5/100 = 0.05
- Therefore, 0.050 x 100 = 5 grams of NaCl.
- Another example is:
- 5 grams of Na2SO4 dissolved in 95 grams of water (roughly 95 mL). Total weight of 100 grams (solute + solvent).
What is Weight/volume (weight per unit volume)?
- This is an expression of weight (mass) per unit volume (W/V), which is often used when a solid chemical is diluted in liquid. Weight per unit volume is expressed as g%.
- It is grams per dL (g/dL), milligrams per dL (mg/dL), or µg/dL. In this case, SI units are weight per µL, or liter (L).
- Examples:
- This is usually expressed as gram/100 mL of diluent.
- To make a 10 % solution, add 10 grams of the substance to a final volume of 100 mL of liquid.
- If you want to prepare 100 mL of 100 g/L of NaCl.
- Weigh 10 g of NaCl and dilute to volume in a 100 mL flask.
What is the Volume/volume (volume per unit volume V/V) solution?
- This is convenient for the composition of two liquids.
- Example:
- If you want to make 50 mL of 2% HCl.
- Calculation:
- 0.20 x 50 = 1 mL
- Therefore, 1 mL of HCL is added to 49 mL of water.
- Calculation:
- 5% of the glacial acetic acid solutions:
- 5 mL of glacial acetic acid diluted with distal water to a volume of 100 mL.
What is a Molar solution?
- This is defined as units of moles per liter (mol/L).
- The symbol M indicates molarity is replaced by mol/L
- or millimoles/millimeter (mmol/mL).
- 1 Mol of a substance = gram molecular weight of that substance.
- Example:
- Make up 250 mL of a 4.8 molar solution of HCl.
- HCL molar weight = 36.5 g.
- 36.5 HCL/mol x 4.8 mol HCL/L x 250/1000 mL = 43.8 g HCL
- 250 mL H2O + 43.8 = 4.8 Molar solution.
- The One molar solution of H2SO4:
- It contains 98.08 g/L of the solution.
- Example:
What is the Normal solution?
- Normality (normal solution) is a gram equivalent weight per liter (eq.wt/L).
- OR milliequivalent weight/milliliter (meq /mL).
- Equivalent weight = gram weight/valency.
- Example:
- NaCl gram weight = 58 gram and valency = 1
- 58/1 = 58 grams equivalent weight per liter.
How will you do Simple Dilution?
- This is defined as the total volume desired and the amount of stock needed.
- The most commonly used equation is:
- V1 x C1 = V2 x C2
- Where V1 is volume, C1 is the concentration of solution 1, and V2 and C2 are the concentration and volume of the diluted solution.
- The basic equation is V1/V2 = C1/C2
- Using the above equation, C1 x V1 = C2 x V2
- Example: Prepare 250 mL of 0.1 M HCL from stock 1 M HCL.
- V1 = unknown
- V2 = 250 mL
- C1 = 1.0 mol/L
- C2 = 0.1 mol/L
Use the formula C1 x V1 = C2 x V2 = Where V1 is unknown.
- V1 = 0.1 x 250 /1.0 = 25 mL
- Measure 25 ml of 1 M HCL; dilute to 250 mL with distle water.
- This diluted solution has a concentration of 0.1 M HCL
- Another Example:
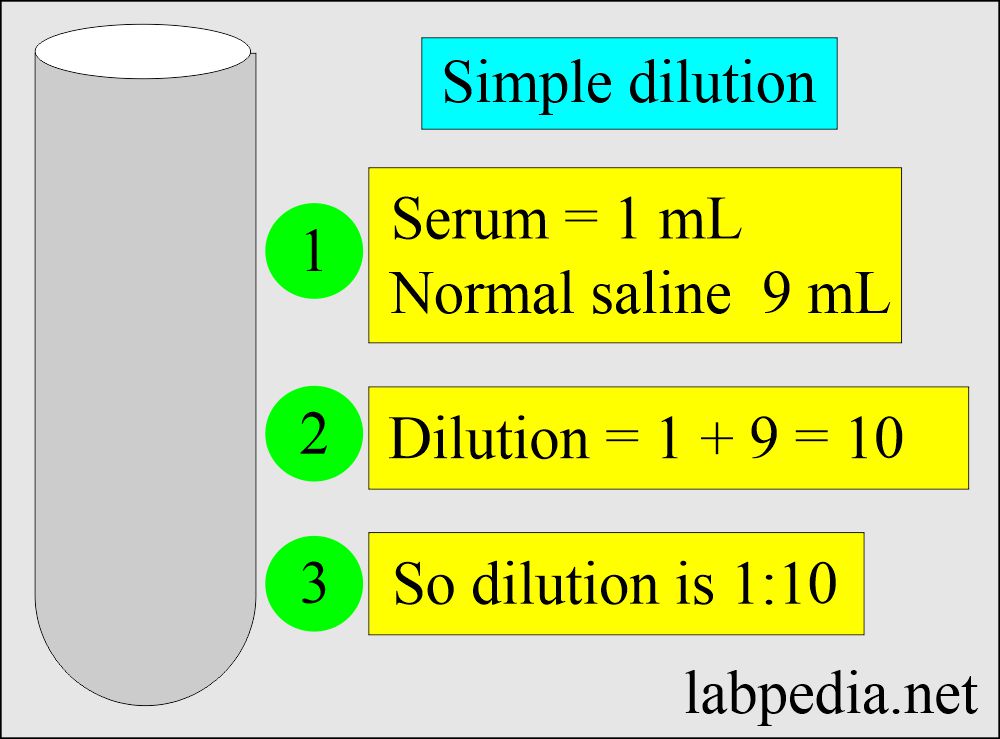
- 1: 10 dilution = ratio of 1 : 9 = 1 : 10 = one part of serum + 9 parts of diluent.
- 100 μL + 900 μL of saline.
- 1 mL serum + 9 mL of saline.
- 2 mL serum + 18 mL of saline.
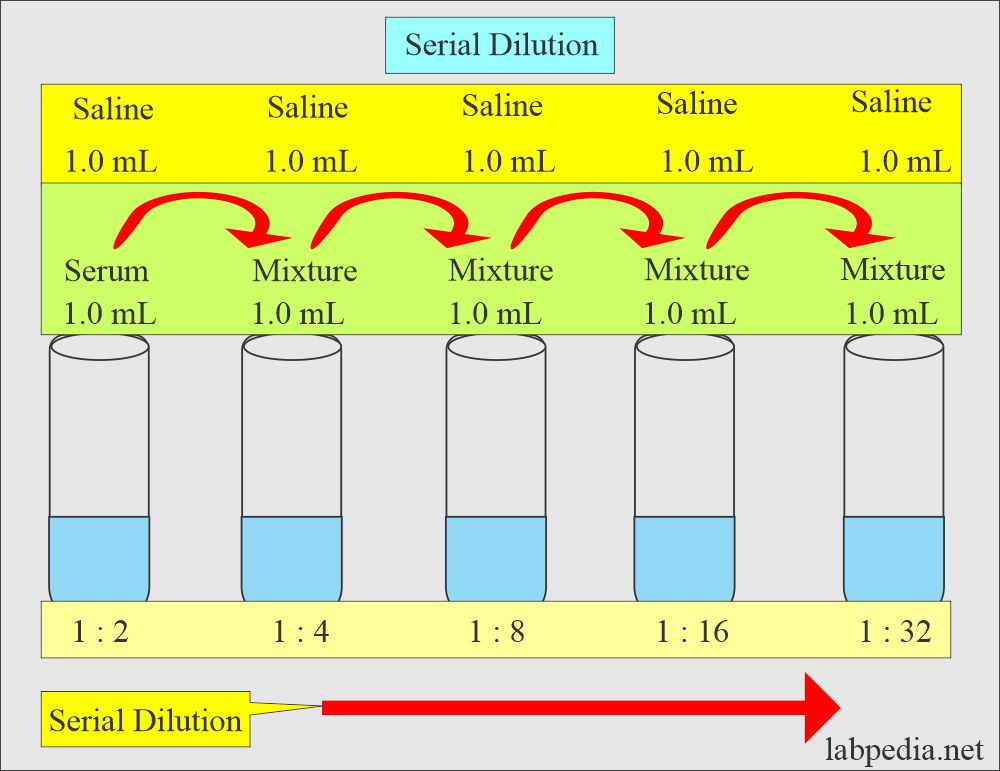
What is Serial Dilution?
- This is defined as multiple progressive dilutions ranging from a more concentrated solution to a less concentrated solution.
- Serial dilutions are useful in a small quantity of serum to find the titer of antibodies.
- The first dilution is made just like the simple dilution.
- Now, subsequent dilutions are made from each preceding dilution.
- If you want to make a serial dilution of 1: 2, 1: 4, 1: 8, 1: 16, and so on.
- The total volume is fixed; suppose it is 1 mL.
- (Initial dilution factor) (next dilution factor) = final dilution factor
- 1 : 2 x 1 : 2 = 1 : 4.
- Procedure
- First tube = (1 mL serum + 1 mL diluent) = 1 : 2
- Second Tube = 1 mL from from ist tube + 1 mL diluent = 1 : 4
- Third tube = 1 mL from tube 2 + 1 mL diluent = 1 : 8
- Fourth tube = 1 mL from tube 3 + 1 mL diluent = 1 : 16
Questions and answers:
Question 1: What is the normal solution?
Question 2: What is the molar solution?


Good
serial dilution of 1: 2, 1: 4, 1: 8, 1: 16
Clear shot it.
Your question is not clear to me. Anyhow I am glad to know that you have clear concept of the serial dilution.