Renal Stone Analysis (Nephrolithiasis), Procedure for stone analysis
Renal Stone Analysis (Nephrolithiasis)
What sample is needed for Renal Stone Analysis?
- Renal stones passed in the urine.
- Renal stones were taken at the time of surgery.
What are the Indications for Renal Stones Analysis?
- This analyses the constituents of renal stones.
- It helps to treat the underlying cause of stone formation.
- For the prevention of future stone formation.
What is the Epidemiology of renal stone formation?
- In the USA, about 5% of women and 20% of men may develop renal stones during their life.
- About 20% of people with stones have elevated serum calcium concentration due to hyperparathyroidism.
- Prolonged deposition causes irreversible damage to kidneys.
- If a stone passes through the ureter or by surgery, it may be checked, and the patient’s diet may be guided.
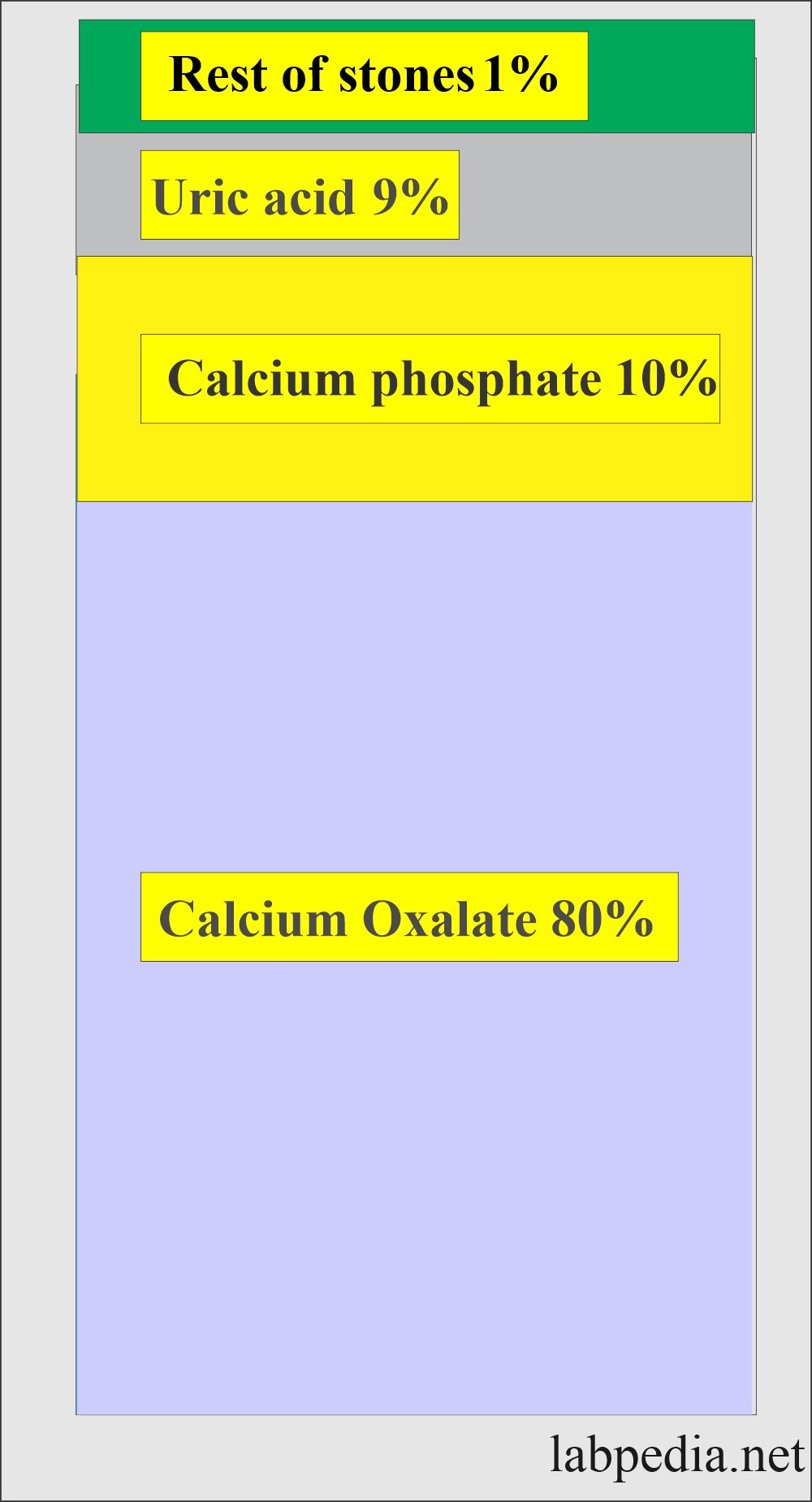
What is the composition of renal stones?
- Calcium oxalate stones are 80%.
- Calcium phosphate is 10%.
- Uric acid is 9%.
- Rest 1% is cysteine, ammonium acid urates, or magnesium ammonium phosphate. Etc.
- These substances crystalize within the organic matrix.
What is the composition of the renal stones?
| Chemicals of stone | % occurrence of the substances in stone |
| Calcium | 97 |
| Phosphate | 88 |
| Oxalate | 65 |
| Magnesium | 25 |
| Ammonium | 20 |
| Urate | 15 |
| carbonate | 12 |
| Cystine | 2 |
| Xanthine | 0.5 |
| Sulfate | rare |
| Cholesterol | rare |
| Iron | rare |
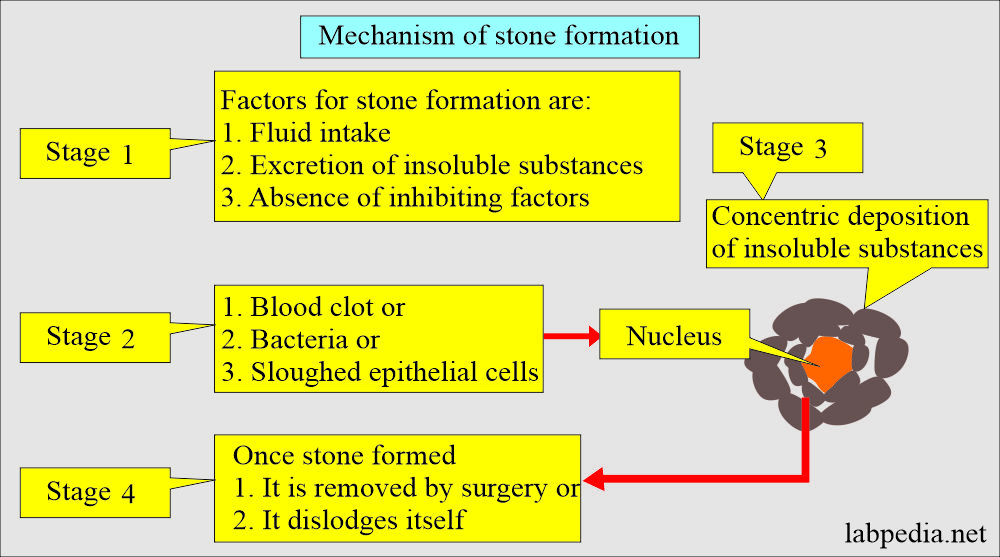
What is the Mechanism of urinary stone formation?
- It depends upon various factors like:
- Urine output depends upon fluid intake.
- Excretion of relatively insoluble substances.
- The absence of a substance inhibits the stone formation.
- Stones form by concentric deposition of the poorly insoluble substance around some nuclei.
- This nucleus may be blood clots, bacteria, fibrin, or sloughed epithelial cells.
- The precipitation of the insoluble substances is initiated by infection, dehydration, urinary obstruction, or excessive intake or production of the compound.
- Once the stone forms, then it starts to grow by accretion unless it is dislodged or removed by surgery.
What is the mechanism of each stone formation?
| Renal stones type | The pH of the urine | Etiology |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What are the factors leading to renal stone formation?
- Renal stone formation promoters are:
- Albumin.
- Globulin.
- Matrix substance A.
- Renal stone inhibitors are:
- Magnesium.
- Citrate.
- Glycoprotein (Tamm-Horsfall).
- Pyrophosphate.
- RNA.
- Predisposing factors are:
- Pre-urinary is:
- More common in the female sex.
- Hot climate. This is due to decreased fluid intake.
- Stress.
- Immobilization.
- Protein-rich diet.
- Urinary factors are:
- Increased Calcium.
- Increased oxalate.
- Increased urate.
- Decreased citrate.
- Decreased Magnesium.
- Increased pH.
- Decreased volume.
- Metabolic abnormalities are:
- Milk-alkali syndrome.
- Renal tubular acidosis.
- Primary hyperparathyroidism.
- Cushing’s disease.
- Hereditary hyperoxaluria.
- Medullary sponge kidney.
What is the role of pH in renal stone formation?
- Calcium oxalate is the most common stone, and it forms in 80% of the population when urine is acidic (low pH).
- Calcium phosphate stone forms when urine is alkaline (high pH).
- Uric acid is 5% to 10%, and it forms when urine is persistently acidic.
- Cystine stones are rare and are a genetic disorder.
Calcium oxalate stones:
How will you discuss the calcium oxalate stones?
- Calcium oxalate stones are the most common. They may be associated with concentrated urine or consistently increased urinary calcium or oxalate excretion.
- Kidney stones may be as small as 1mm in diameter and larger than 2.5 cm in diameter.
- Sometimes, small stones may pass into the ureter from the kidney and from there to the urinary bladder and, ultimately, through the urethra, which goes out.
- Stones produce obstruction and pain.
- 90% of the stones are seen on X-Ray.
- What is the mechanism of Calcium Oxalate stone formation?
- There is a possibility that a person is excreting an excess of Calcium or oxalate.
- Or there is a very small amount of citrate, which binds the calcium and does not form the stone.
- An inherited tendency to absorb more than the normal amount of calcium from the diet leads to hypercalciuria.
- Taking food with a high concentration of calcium or oxalate increases the amount of these substances in the urine.
- Inflammatory bowel disease or intestinal surgery may lead to a nutrient imbalance of absorption and may result in excess urinary calcium.
- A raised parathyroid hormone level leads to an increased calcium level in the urine and blood.
- Metabolic disorders leading to metabolic acidosis cause increased calcium excreted in the urine.
Calcium Phosphate stone formation:
- This has the same factors as calcium oxalate stones.
Uric acid stone formation:
What is the mechanism of uric acid stone formation?
- This is due to increased production of uric acid or excess secretion, seen in the following conditions:
- Gout.
- Disorders of uric acid metabolism.
Cystine stone formation:
What is the mechanism of cystine stone formation?
- This is due to increased production and excretion of cystine, a hereditary disease.
How will you do the renal stone analysis?
Physical examination:
- Describe the shape of the stone.
- Weigh and measure the size.
- Note the color, surface appearance, and consistency.
What will be the appearance of calcium stones?
- Calcium stones = 65%.
- Calcium oxalate stones = 30%
- calcium phosphate = 10%
- Calcium mixed (oxalate+phosphate) = 25%
- These are hard, small to medium sizes, and often multiple.
- An X-ray will show the radiopaque shadow.
What will be the appearance of uric acid stones?
- These are 5%.
- These are small, yellow, and friable.
- These may be large staghorn shapes.
- On the X-ray are radiolucent.
What will be the appearance of magnesium-ammonium phosphate?
- These are 25%.
- These may be large.
- These may be staghorn.
- These are on X-ray are radiopaque.
What will be the appearance of Cystine stone?
- These are 2%.
- On X-ray radiolucent.
- These may be brown.
- These may be large and may be staghorn.
What Reagents are needed for renal stone analysis?
- Sodium carbonate solution.
- 2 mol/L. Dissolve 20 grams of Na2 CO3 in 100 mL of distal water.
- Phosphotungstic acid.
- 50 grams of molybdenum-free sodium tungstate in 350 mL of water, add 20 mL of 85% H2PO4, and reflux (the process of boiling a liquid so that any vapor is liquefied and returned to the stock) for 2 hours.
- Add 1 drop of liquid bromine, cool to room temperature, and makeup to 1 L volume with water.
- Ammonium Molybdate in HNO3.
- Dissolve 5 grams of ammonium molybdate in 123 mL water and add 12 mL concentrated HNO3 with stirring.
- Sodium cyanide, 50 grams/L.
- Dissolve 5 grams NaCN in 100 mL water and add 0.2 mL in concentrated NH4OH.
- Ammonium hydroxide in concentrated form.
- Nitric acid (HNO3) concentrated is needed.
- Sulphuric acid.
- Concentrated H2SO4 is needed.
- Hydrochloride (HCL).
- 1 mol/L. Add 8 mL concentrated HCl to water and make up to 100 mL volume.
- Sodium nitroferricyanide.
- Dissolve 5 grams in 100 mL. Discard when the color fades.
- Ammonium Thiocyanate.
- Dissolve 3 grams in 100 mL water.
- Sodium oxalate.
- Add 5 grams of salt to 100 mL water to saturate it with sodium oxalate, shaking well and allowing it to settle.
- Sodium hydroxide (NaOH) 5 mol/L.
- Dissolve 20 grams of NaOH in water and make it up to 100 mL volume when it is cool.
- Manganese Dioxide (MnO2) powdered reagents.
- Titan yellow.
- Dissolve 0.1 grams in 100 mL water. Add 3 drops (5 mol/L) to make it alkaline.
- Store it in an amber bottle and prepare it every 30 days.
- Alkaline Hypochlorite.
- 0.5 mol/L NaOH, and 30 mmol/L of NaOCl.
- When the solution cools, dissolve 25 grams of NaOH in 600 mL water, then add 43 mL of commercial-grade Hypochlorite solution (52 grams/L NaOCl).
- The reagent is stable for at least 3 months when protected from light and stored at 4 to 8 °C.
- Phenol-Nitroferricyanide.
- 0.5 mol/L phenol and 0.8 mmol/L sodium nitroferricyanide.
- Add 50 grams of reagent-grade phenol to 500 mL water in a flask and 0.25 grams of sodium nitroferricyanide.
- Dilute to the mark with water.
- The reagent is stable for at least 2 months when stored at 4 to 8 °C.
- 0.5 mol/L phenol and 0.8 mmol/L sodium nitroferricyanide.
- Sodium nitrite (NaNO2).
- 0.1 gram/dL in water.
- Prepare just before use.
- N-(1-naphthyl) Ethylenediamine Dihydrochloride.
- Dissolve 0.1 gram /100 mL of the above reagent in water.
- Prepare fresh solution on the day of the procedure.
- Acetic anhydride.
- Chloroform.
- p-Methylaminophenol sulfate.
What is the Procedure for renal stone analysis?
- If the stone is larger than 25 mg, it is pulverized in the mortar and crushed in a test tube.
- If this is less than 25 mg, crush it in a test tube with a glass rod.
- Dissolve the stone in 1 mol/L HCl.
- Add 2 mL of HCl 1 mol/L, and stir to dissolve.
- Take the supernatant for the analysis.
How will you find Calcium?
- 3 drops of the supernatant on a slide or plate.
- 4 drops of sodium oxalate.
- 2 drops of NH4OH.
- Result: White precipitate positive for calcium.
How will you detect Oxalate?
- Take a pinch of MnO2 and add it to the supernatant fluid.
- If there is the release of tiny gas bubbles.
- Result: Positive for oxalate.
How will you detect Urate?
- Stone powder or residue adds one drop of Na2CO3.
- Add 2 drops of phosphotungstate solution.
- Result: Positive prompt blue color develops.
How will you detect Carbonate?
- Observe the supernatant fluid for bubbles when the acid is added.
- Result: Positive when you see effervescence.
How will you detect Phosphate?
- Place a few mg of the crushed stone on the plate (or slide).
- For very small stones can use 3 drops of the supernatant.
- Add 2 drops of the ammonium molybdate.
- Add 2 drops of p-methylaminophenol sulfate.
- Result: Positive deep blue color.
- You can run the blank (negative control) with the test.
- Result: Positive deep blue color.
How will you detect Magnesium?
- Place 3 drops of the supernatant on a white spot plate or slide.
- Add 3 drops of NaOH.
- Add one drop of Titan yellow solution.
- Result: Positive when seeing a red precipitate.
How will you detect Ammonia?
- Take 3 drops of the supernatant off the white spot plate or slide.
- Add 2 drops of NaOH.
- Add one drop of phenol-nitroferricyanide solution.
- Add one drop of alkaline hypochlorite solution.
- Mix and place in a 37 °C incubator for 3 to 5 minutes.
- Result: Positive blue color develops.
How will you detect Cystine?
- A few mg of stone powder on a spot plate or slide.
- Or take the residue from the acid solution.
- Add one drop of NH4OH.
- Add one drop of NaCN.
- Wait for 15 minutes.
- Add 2 drops of sodium nitroferricyanide.
- Result: Positive is a brick red color.
How will you detect Cholesterol?
- Take small powder in the small test tube.
- 5 drops of chloroform and stir it.
- Add 10 drops of acetic anhydride.
- One drop of concentrated sulphuric acid.
- Result: Positive when bluish-green color develops.
How will you detect Xanthine?
- Place the stone powder in the evaporating dish.
- Add 0.5 mL of concentrated HNO3.
- Heat to dryness in the fumes hood.
- Add concentrated NH4OH to a residue in the dish.
- Result: Positive when the yellow residue turns orange on the addition of NH4OH.
How will you detect Iron?
- Make powder of the stone.
- Add 3 drops of HNO3.
- Add 3 drops of NH4SCN.
- Result: Positive when red color develops.
How will you follow the patient with stone formation?
- Other tests needed to evaluate the renal stone formation include:
- Urinalysis to see the pH, presence of RBCs or WBCs, and type of crystals.
- Blood and Urine were collected for 24 hours to evaluate the amount of calcium, uric acid, and creatinine. It can evaluate oxalate, phosphates, citrate, or cystine.
- Complete blood count to rule out acute infection.
How will you diagnose stones in a patient?
- Urine analysis.
- Ultrasound.
- CT scan.
- A blood test is used to guide the patient in the formation of stones.
What is the outcome of renal stones?
- <5 mm in D stones have a high chance of passing out.
- Stones 5 to 7 mm in diameter have a 50% chance of passing out.
- Stones of >7 mm in diameter need treatment.
Questions and answers:
Question 1: At what size of the stone intervention is needed?
Question 2: At what pH uric acid stone will form?