Quality Control (QC) of the Clinical laboratory
Quality Control (QC)
- The principles of quality assurance, quality control, and quality management are the foundations for good laboratory results and work.
- Every laboratory test produces a result. However, until the result is verified by some means, it is not possible to be sure about its accuracy. Quality control is what will give confidence about the result.
- The technicians have to be sure of the accuracy of the test results.
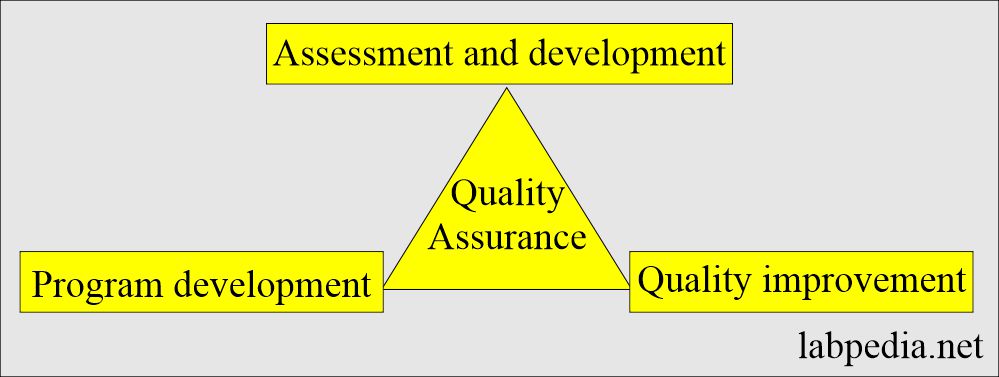
How will you define Quality assurance (QA)?
- It consists of plans, policies, and procedures that provide an administrative structure for a laboratory’s efforts to achieve quality goals.
What is essential about Quality assurance (QA)?
- It has three components:
- Assessment and monitoring.
- Development of the program.
- Quality improvement (quality control).
- Quality assurance is vital to the patients, and this will need:
- Quality can be assessed and monitored.
- Quality program development.
- Quality control improvement.
- Quality control is one component of quality assurance. Quality assurance will check the entire testing process and will check quality regularly.
- Quality can be assessed and monitored, and it can be improved.
How will you define Quality control (QC)?
- It will ensure the accuracy and reproducibility of the lab’s various tests.
- QC checks the particular source of errors, estimates the magnitude of the errors, and alerts the laboratory personnel that quality has deteriorated.
- Quality control results will be acceptable when these are within the acceptable range of error limits.
- Quality control (QC) results are unacceptable when these results show excessive errors and are out of the range.
- Quality control goals are:
- Accuracy.
- Precision.
- The total error of the chemical method.
What are the ideal properties of quality control (QC) materials?
- Quality control (QC) material should resemble human serum, plasma, blood, urine, and cerebrospinal fluid.
- Quality control (QC) material should be stable without interfering with preservatives.
- Quality control (QC) material should be free of communicable diseases like bacteria, viruses, and fungi.
- Quality control (QC) material should have a known concentration of the analytes.
- Quality control (QC) material should be easy to store and dispense.
- Quality control (QC) material needs to be affordable and not too costly.
What are the Quality control (QC) objectives?
- Quality control (QC) ensures continuous accuracy of results.
- Quality control (QC) gives an early warning about the accuracy of the test so that early remedies may be taken to avoid great mistakes.
- Quality control (QC) compares the tests simultaneously from the same control sera at different times.
What are the tools used for quality control (QC)?
- Procedure manuals.
- Maintenance schedules.
- Calibrations.
- Quality assurance program.
- Training.
What are the Quality control (QC) for different disciplines of pathology?
- Quality assurance for the blood transfusion.
- Quality assurance for microbiology.
- Quality assurance for biochemistry.
- Quality assurance for surgical pathology.
- Quality control for hematology.
What are the factors on which Quality control (QC) is dependent?
- The time between the collection and the performance of the test, e.g.
- Leukocytes and RBCs utilize glucose and cause a steady decrease in glucose concentration.
- Specimen storage also causes an error in the result.
- Evaporation of the sample may cause the wrong result, such as electrolytes.
- Exposure to light affects the Bilirubin level.
- Refrigeration will affect lactate dehydrogenase (LDH).
- Clerical mistakes may occur at any stage.
What is the basic purpose of Quality control (QC)(?
- To maintain a continuous record of the precision of the tests.
- It gives an early warning of the control trends, and early action will prevent a major mistake.
- It provides valid judgment on the accuracy of results by comparison with the known sera.
- This is very important for automated analyzers to check their performance.
- Monitor the analytic process and help to find which method is more accurate.
- This also helps to evaluate the technologist’s skills.
- Determine analytical errors during analysis.
- Prevent incorrect patient values.
- The monitoring of the analytic values is compared with known standards and their expected values.
What are the various terms of Quality control (QC)?
- Accuracy means the true value of the analyte. Determining the true value of a substance isn’t easy.
- The value, in comparison to the known control, has some advantages.
- Precision measures reproducibility for a particular test. A method may give excellent precision but poor accuracy.
- Mean is a basic statistical work, where this is the mean of the sum of data divided by the number of items.
- The mode is the value that occurs most frequently in a list of data items. It is not affected by extreme values.
- Standard deviation is a mathematical concept. It is very important because the mean values are not acceptable if they are outside of a standard deviation of >2.
- Median: When the data is arranged in an ascending or descending manner, the number that occupies the central position is the median.
- Centile is the percentile; the value is greater than a specified percentage of the list of values.
How will you minimize the Analytic factors, which depend upon instrumentation and reagents?
- A schedule of daily and monthly preventive maintenance is needed for each instrument.
- Check water quality, power supply, electrical balance, and glassware and pipette calibration.
- Reagents and kits should be dated when received and when opened.
- Run new lots of the reagents with the old lot in parallel before using them for analysis.
- The primary standard is the most highly purified substance.
- The secondary standard is one whose concentration is determined by analysis and compared with the primary standard.
- Post-analytic errors are due to the recording and reporting of the results.
What are the Analytic errors?
- Random errors affect precision and are the basis for varying differences between repeated measurements.
- The systematic error component indicates a constant difference, either increased or decreased. This may be caused by:
- Poorly made reagents or standards.
- Instrumentations defects
- Poorly written procedures.
What are the characteristics of Quality control (QC) material?
- Available in sufficient quantity.
- It should be stable for a period of a minimum of one year.
- Keep in small volumes.
- Its concentration should vary minimally.
- Their composition should not vary from vial to vial.
- Control material, such as the test sample, should be tested.
What is the External quality assessment program?
- This is the program in which the specimens are subjected to other laboratories for analysis, and the individual laboratory results are compared.
What is the focus of Total quality management?
- Customer. The users are doctors and nurses, while the customers are patients.
- Management commitment.
- Training of the workers.
- Measurement through quality-improved tools.
- Quality improvement occurs when the problems are permanently eliminated.
- Issues arise from imperfect procedures, of which 85 %.
- The remaining 15% of cases need action and performance improvements of individual employees.
- So, the main problems are management problems, and management has the power to change the work process.
How will you control the preanalytical mistakes in Quality control (QC)?
- Patient identification and labeling are critical. Barcode technology has reduced these mistakes, which are common in handwritten labels.
- Keep a record of the sample received and when the report is ready.
- Check the request form, the test tube name, and the requested tests.
- Check the adequate amount of the sample.
- Observe if there is hemolysis or lipemic serum.
- Take the history of food intake, alcohol, drugs, smoking, stress, sleep, and posture because these factors may influence the result.
- Explain all instructions to the patient for the collection of the sample.
- Incorrect containers and incorrect preservatives will affect the result.
- Transport of the sample is very important and may be critical to some of the tests.
- Processing the sample as a separation of the serum, where centrifuge speed, temperature, and the person are important.
What are the errors in quality control (QC)?
- Quality control errors in the laboratory are classified into:
- Random errors:
- These mistakes will increase the standard deviation.
- These are present in pipettes and volumetric glassware with manufacturing defects.
- There may be a defect in the instruments and spectrophotometer.
- There may be a defect in the cuvet temperature.
- Light, temperature, and evaporation may affect the serum or plasma analyte values.
- There may be interference from other substances in the analyzed sample.
- Clerical errors:
- These are unavoidable and should not be accepted.
- Labeling the wrong name of the patients.
- Delay in the transport of the sample.
- Incorrect calculations.
- These can be avoided by:
- Well-trained staff.
- By good working organization.
- Well-designed worksheets.
- Thorough checking of the results.
- Systemic errors:
- These errors will displace the mean value in one direction, which may go up and down.
- There may be instability of the reagents.
- There may be an inaccuracy in the standards.
- If the method is nonspecific for the analysis.
- Analysis of analytes by the kinetic method at 340 nm is critical.
- Some errors encounter both systemic and random errors.
Questions and answers:
Question 1: What are the tools of quality control?
Question 2: Why is quality control important?


Wonderful site for quality assurance
Thanks for the remarks.
Superb info about Q.Control.
Thanks.
How to set tha control values
You can use either ready-made commercially available standards. Another easiest way is to take sera from the different samples (received in your lab from different patients), mix the sera, and check the values of the tests you want. Make aliquotes of this serum and can freeze it. Daily this serum you can be run as a control.
WHY QUALITY CONTROL SAMPLES ARE TURN TO GREEN COLOUR AFTER 4 HOURS OF PROCESS COMPLETE.
Any idea about it?. Which reagent interfere is this colour change. Kindly suggest ur valuable comment.
As I know, once you reconstitute the quality control standards commercially available, you have to make aliquotes and freeze them. As per principle, you should consume that standard control within few hours. You have not mentioned which company standard you are using. Read its literature thoroughly. They might have explained the color change.
Can msc analytical chemistry student work in qc department in clinical research organization
I think you have to talk to the concerned department.
Why we should keep high level for qc
A high level of QC is important to get the correct results.
A very clear explanation about qc. Thank you so much sir
Thanks
Thanks.
😀