Alcohols: Ethyl alcohol (Ethanol), Methanol, Isopropanol, Ethylene Glycol, and Their Complications
Ethyl alcohol (Ethanol)
What sample is needed for Ethyl alcohol (Ethanol)?
- Alcohol levels can be estimated in blood, breath, and saliva.
- When collecting blood, don’t clean the site with alcohol. Clean the site with an alcohol-free disinfectant like benzalkonium chloride (Zephiran).
- A blood test is the best sample for the estimation of alcohol.
- Blood samples in a living patient may be whole blood, serum, or plasma.
- The serum and blood alcohol ratio is 1:14.
- Blood in a cadaver is taken from the aorta.
- The blood must be capped to avoid the evaporation of alcohol.
- Collect blood in sodium fluoride or potassium oxalate.
What are the precautions for Ethyl alcohol (Ethanol)?
- Use alcohol-free disinfectants. Take a blood sample without the use of any alcohol skin-cleaning solution.
- Alcohol is volatile, so cap the bottle to avoid evaporation.
- The blood can be stored properly and sealed for 14 days at room temperature or 4 °C with or without preservatives.
- For longer storage or nonsterile postmortem material, specimens use preservative sodium fluoride.
How will you measure alcohol levels in various samples?
- Blood alcohol levels were tested in serum or plasma or whole blood.
- An arterial blood sample is higher than a venous sample.
- Capillary blood from the finger prick or ear lobules is about 70% to 85% of the arterial blood.
- The serum value is 18% to 20% higher than the whole blood level.
- Blood levels correlated by the law are whole-blood samples.
- Serum: whole blood ratio is 1.03 to 1.35.
- Rainey, in 1995, gave a median serum/whole blood conversion ratio of 1.15 rather than 1.20.
- Breath test:
- The police mostly use breath tests; these are easy and can be done anywhere.
- A breath analyzer measures the ethanol concentration at the end of deep expiration after the deep inspiration.
- Alcohol level: breath/blood alcohol ratio is 2100:1. Multiply the value by 2100.
- Breath alcohol = g/210 L
- The above value is equal to blood alcohol g/dL.
- Before performing the breath test, wait for 15 minutes to rule out:
- Alcohol may be present in the mouth in case of recent drinking.
- Vomiting containing alcohol-rich gastric fluid.
- Alcohol-containing mouthwash.
- There should be no smoking.
- Some mouthwashes produce a significant level 2 minutes after use, but it disappears after 10 minutes.
- Ketone bodies may interfere with the breath test.
- In case of a negative breath test, indicate some other medical emergencies.
- Saliva may be used where alcohol concentration is 9% higher than that in the whole blood.
- This is an easy and noninvasive method.
- The urine alcohol sample is noninvasive and easy to collect.
- During the post-absorptive stage after alcohol intake, the concentration of alcohol in the urine is roughly 1.3 times that in the blood.
- This is variable, so emptying the bladder and collecting urine after 20 to 30 minutes is better.
- It can detect the ingestion of alcohol within the previous 8 hours.
- Urine samples are not recommended because of the variable blood/urine ratio and the stasis of the urine in the urinary bladder.
- However, urine can be used for screening purposes.
What are the Indications for Ethyl alcohol (Ethanol)?
- This test is done on the drivers.
- Quantitation of the alcohol level is done for therapeutic or legal purposes.
- The alcohol level test is done to diagnose alcohol intoxication.
- Alcohol levels may be done in cases of coma, cerebral trauma, and drug overdose.
- To differentiate an alcoholic intoxication coma from a diabetic coma.
- This test is also done for alcoholism.
How would you define an alcoholic person?
- Alcohol shows a range of actions on the central nervous system, extending from sedation to anesthesia, and intake of alcohol leads to impairment of judgment and thinking and altered behavior.
- The legal definition of alcohol intake is when a person has a blood level of alcohol of 0.1 g/dl or 100 mg/dL.
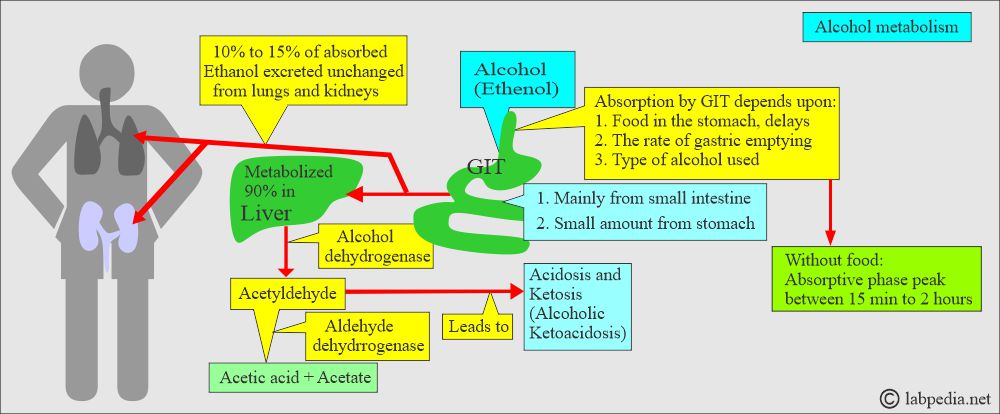
- Alcohol is Ethanol, and it is readily absorbed from the GI tract.
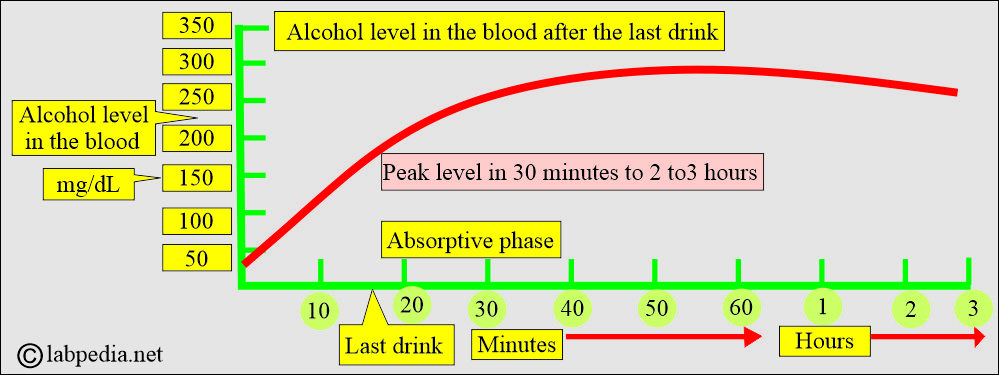
- The peak level is within 40 to 70 minutes after the intake.
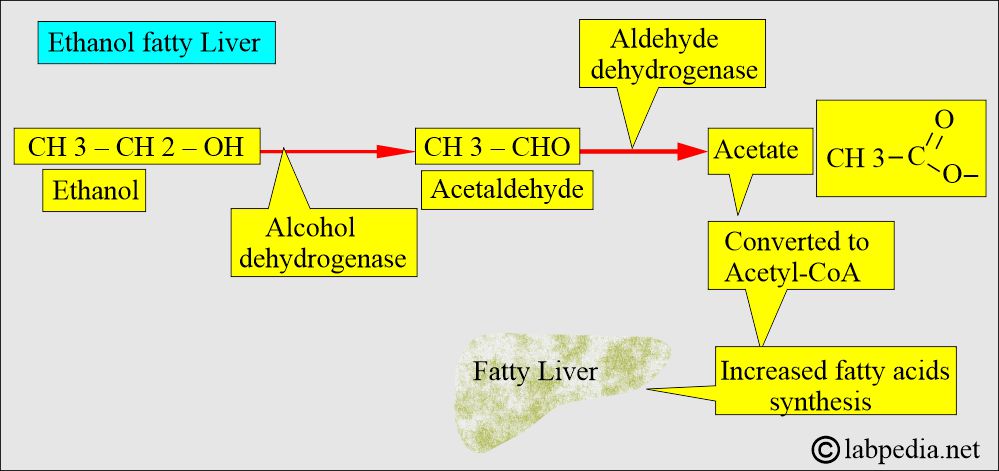
- The liver enzyme dehydrogenase metabolizes ethanol into acetaldehyde.
- 90% of alcohol is metabolized in the liver.
- This acetaldehyde is converted into acetic acid by the enzyme Aldehyde Dehydrogenase.
- How will you diagnose alcoholism?
- A major criterion is a blood alcohol level >15 mg/dL at any time.
- The minor criterion is blood alcohol concentration is >300 mg/dL at any time and blood alcohol concentration is 100 mg/dL.
- Toxic concentration blood alcohol level is ≥200 mg/dL.
- The lower limit to detect blood alcohol level is 100 µg/mL.
How would you discuss Alcohol (Ethyl alcohol) metabolism?
- Ethanol easily diffuses into bodily fluids and is partially cleared in the urine and other bodily excretions.
- Its major metabolic pathway is its conversion into acetaldehyde by the alcohol dehydrogenase enzyme in the liver.
- Acetaldehyde is converted into acetate by the acetaldehyde dehydrogenase enzyme.
- Ethanol metabolite acetaldehyde leads to acidosis and ketosis called Alcoholic ketoacidosis.
- Ethanol is converted to acetaldehyde.
- Headache, flushing, and hangover are due to acetaldehyde before it is metabolized to acetate.
- Once the peak level is reached, then its level decreases.
What are the effects of Ethyl alcohol (Ethanol) on the body?
- Ethanol depresses the CNS and may ultimately lead to coma and death.
- ≤50 mg/dL = Euphoria and decreased inhibitions.
- 100 to 300 mg/dL = Incoordination and decreased orientation.
- >400 mg/dL = Coma and death.
- CNS dysfunction is more pronounced when:
- Absorptive phase: Ethanol concentration in the blood increases.
- Elimination phase: When the level of alcohol is declining.
- Alcohol causes diuresis by inhibiting the secretion of the ADH (antidiuretic hormone) by the posterior pituitary.
- It also inhibits the secretion of oxytocin because this property is used to stop uterine contractions in premature labor.
- An alcohol blood concentration level of 100 mg/dL has been established to limit car/truck driving in most states in the United States.
-
- While in 17 states, this limit is 80 mg/dL.
-
- When ethanol is used with other CNS-depressant drugs, then ethanol exerts a potentiation or synergistic depressant effect.
- Alcohol is present in the blood, urine, stomach contents, and breath.
- Saliva’s alcohol level is 9% higher than blood.
- The blood-alcohol level of 50 to 100 mg/dL causes:
- Slowing of reflexes.
- Flushing.
- Impaired vision.
- The blood-alcohol level of >100 mg/dL causes:
- Signs of CNS depression were seen.
- Hypotension.
What are the toxic effects of blood alcohol?
- A blood alcohol level >300 mg/dl is usually associated with a coma.
- A blood-alcohol level of >400 mg/dL is fatal, and death may occur.
- Pregnant ladies who drink alcohol will have low-birth-weight babies.
- Alcoholic mother babies will have mental retardation and fetal alcohol syndrome (an irreversible congenital disorder).
- Nutritional and metabolic studies have demonstrated that alcohol can cause hypertension.
How does the absorption of the Ethyl alcohol (Ethanol) take place?
- The blood-alcohol level will increase roughly 15 to 25 mg/dL when adults take:
- An ounce of whiskey or.
- 12 ounces of the bear or.
- One glass of wine.
- Women absorb more quickly than men and show a 35% to 45% higher blood alcohol level.
- During the menstrual cycle, the peak occurs more rapidly. Birth control pills cause higher levels and sustain them.
- The use of sedatives like barbiturates and benzodiazepines with alcohol is hazardous and may lead to death by respiratory depression.
- In old age, toxicity develops more quickly than in adults.
What is the rate of elimination of ethanol from blood circulation?
- Men = 11 to 22 mg/dL/hour.
- The average level is = 15 mg/dL/hour.
- Women = 11 to 22 mg/dL/hour.
- The average level is = 18 mg/dL/hour.
- Drinking habits also influence the elimination rate.
- For example, = Alcoholics have an average elimination rate of about 30 mg/dL/hour.
What are the complications of alcohol in chronic drinkers?
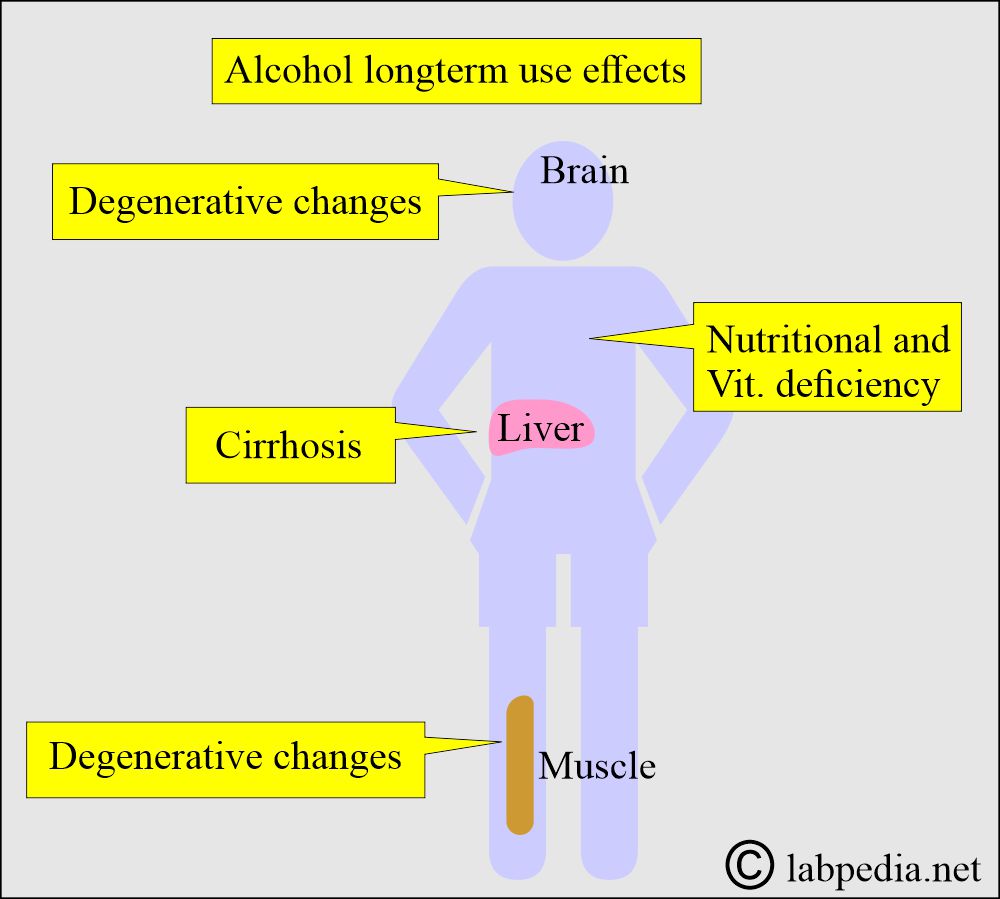
- Chronic use of alcohol may lead to the following:
- Cirrhosis of the liver.
- The degenerative changes in the brain.
- The degenerative changes in the skeletal muscles.
- Chronic alcoholics may have nutritional and vitamin deficiencies.
- Ethanol ingestion leads to hypoglycemia and ketonemia because of the inhibition of gluconeogenesis.
- Lactate accumulates and competes with uric acid for excretion through the kidneys, increasing the serum uric acid level.
- When alcohol is taken with fatty meals, it leads to hypertriglyceridemia, which may persist for more than 12 hours.
- A moderate intake of alcohol for one week leads to increased serum triglyceride >20 mg/dL.
- The toxic level of alcohol stimulates the release of:
- Cortisol.
- Catecholamines.
- Increased intake of alcohol leads to:
- The decreased plasma testosterone level in the men.
- There is an abnormal pituitary, adrenocortical, and medullary function.
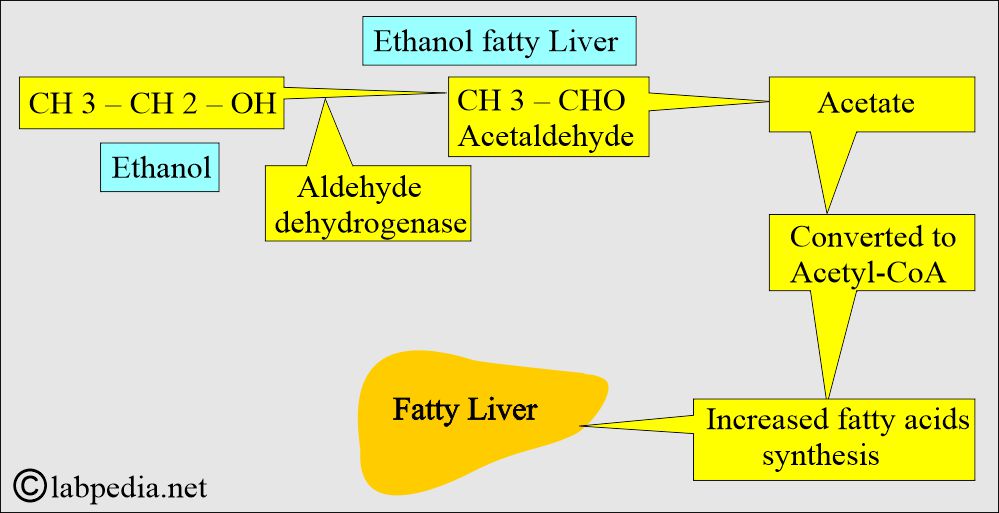
- Alcohol ingestion, after metabolization, leads to acetaldehyde formation, which causes damage to the mitochondria of hepatocytes, and H+ leads to fat accumulation.
- In chronic alcoholism, there is an increased level of acetaldehyde and acetate.
- The acetate enters the acetyl-CoA cycle and leads to increased synthesis of fatty acids, resulting in fatty liver.
- Ethanol leads to diuresis by inhibiting the ADH from the posterior pituitary.
- It also inhibits the secretion of Oxytocin from the posterior pituitary. So, it can be used to stop uterine contraction in premature labor.
- When alcohol (ethanol) is not available, people may use other alcohols, such as methanol (methyl alcohol), also called wood alcohol, isopropanol (rubbing alcohol), and ethylene glycol (antifreeze alcohol). Sometimes, these are present as contaminants in the ethanol.
- All these are associated with toxicities.
- Their metabolism has direct toxic effects from their metabolites.
How will you compare various types of alcohol intoxication (poisoning)?
| Type of alcohol |
Metabolic acidosis with anion gap |
Osmolal gap | Urine ketones | Urine oxalate crystals | Serum acetone |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What are the long-term effects of chronic alcoholism?
- Anemia.
- Cancers.
- Cardiovascular diseases.
- Cirrhosis.
- Dementia.
- Depression.
- Seizures like epilepsy.
- Gout.
- Hypertension.
- Increased risk for infections like tuberculosis, HIV, and pneumonia.
- Alcoholic neuropathy.
- Gastritis and pancreatitis.
What are the various levels of alcohol and interpretations?
| Clinical interpretation | Level of alcohol in the blood |
|
|
|
|
|
|
|
|
|
|
| Toxic level | |
|
|
|
|
|
|
|
|
- The lower limit of detection is 10 mg/dL.
- >80 mg/dL is considered positive for driving under the influence in most states.
- >300 to 400 mg/dL is considered fatal.
What are the critical Values?
- >300 mg/dL.
- In a high-dose coma, blood alcohol should be >300 mg/dL; otherwise, rule out diabetic acidosis and hypoglycemia.
- A blood alcohol level of >150 mg/dL without any evidence of intoxication suggests that the alcoholic patient has increased tolerance.
What will be the Clinical presentation of the alcoholic person?
| Blood alcohol level | Patients presentations |
|
|
|
|
|
|
|
|
|
|
|
|
What are the Clinical stages of alcoholic intoxication?
|
Blood alcohol concentration (% weight/volume) |
Urine ethanol (% W/V) | Clinical symptoms of alcoholic intoxication |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- (modified from ASCP )
| The blood alcohol concentration in g/dL | Clinical Stage of alcohol influence | Clinical presentation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
How will you diagnose alcoholism?
- Major criteria:
- Blood alcohol concentration >150 mg/dL without evidence of intoxication.
- Minor criteria:
- Blood alcohol concentration >300 mg/dL at any time.
- Blood alcohol concentration 100>mg/dL in a routine examination.
- The toxic concentration is >200 mg/dL.
- The lower limit for detection is 100 µg/mL.
How will you diagnose alcohol intoxication?
- Blood test:
- Most court of law follows the National Safety Council on alcohol intake.
| The blood level of alcohol | Court of Law Recommendations |
|
|
|
|
|
|
- The American Medical Association (AMA) and the Council on Scientific Affairs (1986) suggest that a 0.05% blood alcohol level suggests alcohol and impaired driving.
- Breath test:
- It is discussed in detail above.
- The breath-to-blood ratio is 0.00048:1.
- Urine test:
- This is not recommended because of the high blood/urine ratio.
- This is also a noninvasive method.
- It is recommended to empty the bladder first and then collect urine after 20 to 30 minutes.
- Saliva ethanol:
- It is easy to collect saliva and is a noninvasive method.
- Saliva ethanol level is about 9% higher than the whole blood.
What are the tests to evaluate chronic alcoholism?
- γ-GT (GGT) will give information about the damage caused by alcohol use and will be raised.
- GGT has a sensitivity of 70% (range maybe 63% to 81%).
- AST (SGPT) has the same role as GGT. It is raised in 50% of the cases (range maybe 27% to 77%).
- MCV detects the deficiency of folic acid. This may be abnormal in 60% of the cases (26% to 90%).
- Other abnormalities found in <40% of the cases are:
- Hypophosphatemia.
- Hypoglycemia.
- Hypochloremic alkalosis.
- Increased lactic acid.
- Hyponatremia.
- Hypomagnesemia.
- Hyperuricemia.
- Hypertriglyceridemia.
- Metabolic acidosis with increased anion gap.
Methanol (Wood alcohol)
- It is used as a solvent in several commercial products.
- Alcoholics use it because of the low price.
- This may be contaminated with the ethanol.
- S/S of CNS is less severe than that of ethanol.
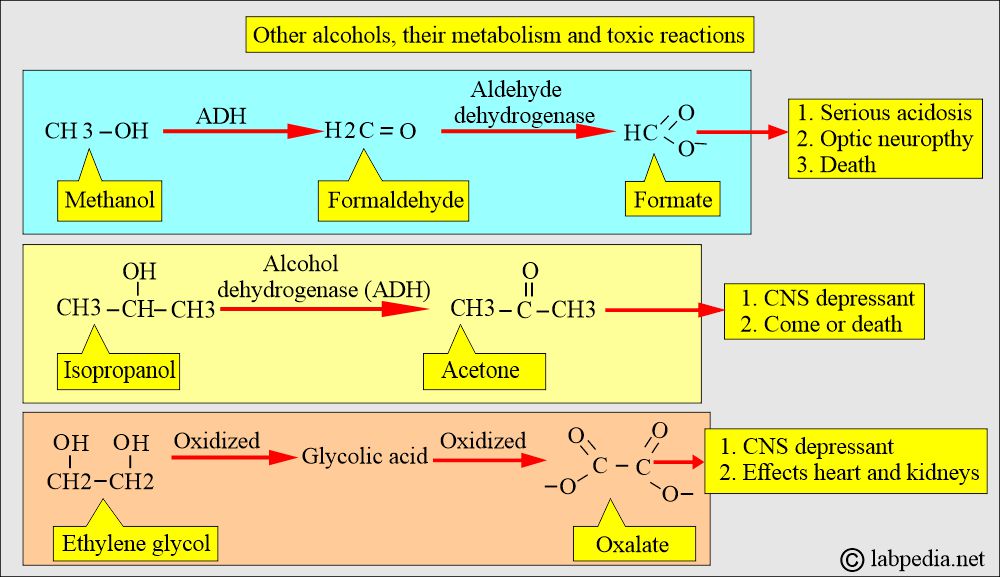
- What is the pathogenesis of toxicity by methanol?
- The liver alcohol dehydrogenase (ADH) enzyme in the liver converts it into formaldehyde.
- Formaldehyde is oxidized rapidly by the aldehyde dehydrogenase to formic acid.
- Formic acid accumulates in the blood and leads to metabolic acidosis, which has a latent period of 12 to 27 hours.
- The patient will have toxicity due to formate (formic acid).
Isopropanol (Rubbing alcohol)
- This is readily available to the general population.
- It is used as rubbing alcohol.
- This has twice the CNS depression effect.
- This is not as toxic as methanol.
- This has a short half-life of 3 to 6 hours due to rapid conversion by alcohol dehydrogenase (ADH) to acetone.
- Acetone is eliminated slowly through respiration and urine.
- Acetone has the same properties as a CNS depressant as ethanol, but it is prolonged.
- Severe isopropanol toxicity may lead to coma or death.
- What is the Toxic level of Isopropanol?
- >400 mg/L = Indicates severe toxicity.
- >1000 mg/L = Patient will go into a coma.
- The presence of acetone in the urine and blood, when it is at a high level, will indicate isopropanol poisoning.
Ethylene glycol
- This is used as an antifreeze. It is also used in the polyester fiber industry.
- This is also CNS depressaant.
- Then, it affects the heart and is followed by the kidneys.
- It is oxidized to glycolic acid and then to oxalate.
- There is severe metabolic acidosis with an increased anion gap.
- One can detect ethylene glycol and its metabolite glycolic acid in the blood (serum).
- Can see oxalate and hippurate crystals in the urine.
- Dialysis is needed if the glycolic acid level is >50 mg/dL.
How will you compare various types of alcohol intoxication (poisoning)?
| Type of alcohol |
Metabolic acidosis with anion gap |
Osmolal gap | Urine ketones | Urine oxalate crystals | Serum acetone |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Questions and answers:
Question 1: At what alcohol level does a person go into a coma?
Question 2: What is toxic level of isopropanol?