Enzymes:- Part 1 – Introduction of Enzymes
Introduction of Enzymes
What sample is needed for enzyme estimation?
- The serum is considered to be the best sample compared to plasma.
- The best sample to prepare the serum is venous blood.
- The plasma may interfere with some enzymes, like acid phosphatase and alkaline phosphatase.
- Capillary blood may show abnormal Lactate Dehydrogenase levels.
- Capillary blood has increased total LD and abnormal LD isoenzymes.
How will you define enzymes?
- Enzymes are protein catalysts in all body cells and catalyze specific reactions. These enzymes make life possible.
- These are proteins, and they work as catalysts, increasing the rate of a chemical reaction.
- The enzyme does not affect the equilibrium point.
- The enzyme enters the reaction but is not consumed.
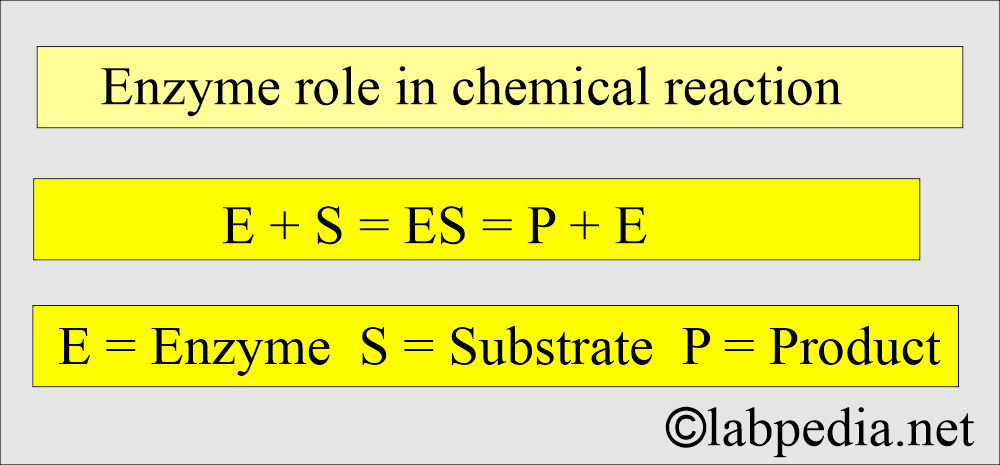
- Enzyme = E, Substrate = S, Enzyme-substrate complex = E+S Product = P
- E + S ↔ E-S ↔ P + E
- Enzyme = E, Substrate = S, Enzyme-substrate complex = E+S Product = P
What are the enzyme’s functions?
Enzymes catalyze all-important chemical reactions like:
- Reduction.
- Oxidation.
- Esterification.
- Hydrolysis.
- Molecular interconversion.
- Synthesis.
What is the role of an enzyme in energy supply?
- The enzyme reaction supplies energy for chemical changes necessary for vital activities like:
- Nerve conduction.
- Muscle contraction.
- Digestion.
- Respiration.
- Reproduction.
- Growth.
- Maintenance of body temperature.
What is the role of the enzyme as a catalyst?
- The enzyme is a catalyst that increases the rate of a particular chemical reaction without being consumed or permanently altered.
- The enzymes will accelerate the rate of reaching equilibrium.
- All enzymes are proteins synthesized in the body, similarly to other proteins.
- Each enzyme synthesis is under the control of a specific gene.
- Life is an integrated series of enzymatic reactions, and whenever a change in enzyme reaction leads to disease.
- Enzymes are a sensitive indicator of pathological changes in any organ.
What is the mechanism of an enzyme reaction?
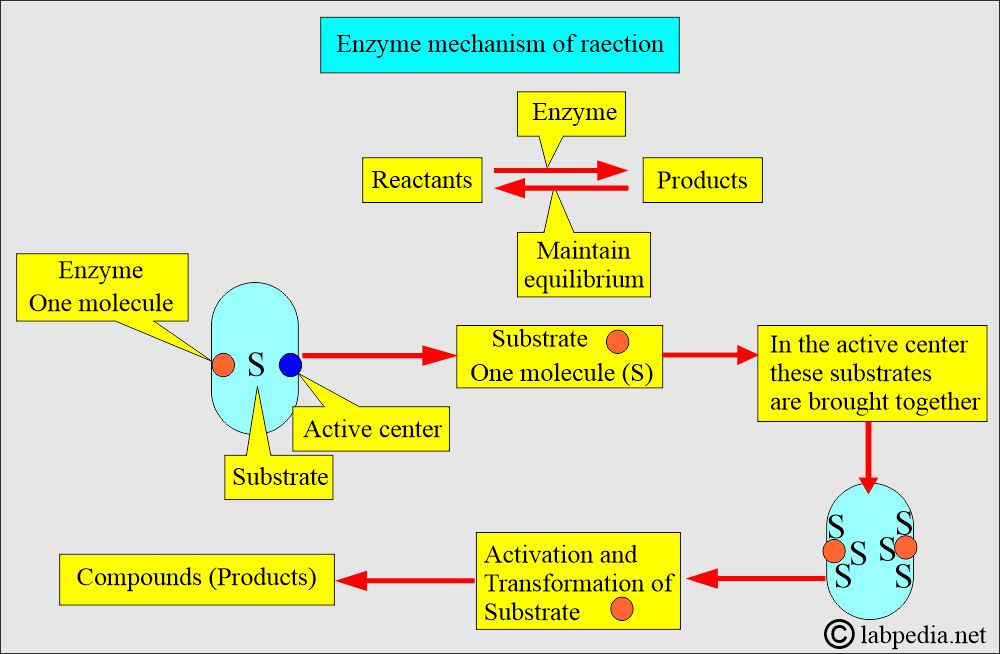
- Enzymes catalyze the conversion of one or more compounds (SUBSTRATES) into one or more different compounds (Products).
- The enzyme molecule has an active center where the substrate combines.
- Substrates are brought together in the active center to activate and transform the substrate.
How will you classify enzymes?
What is the basis of classification?
- Principle of classification: The catalyzed reaction type and the substrate involved identify the enzymes.
- On this basis, these are classified into the following groups:
- Transferases: These enzymes catalyze the transfer of moieties such as glycosyl, methyl, or phosphoryl groups.
- Lyases: It will catalyze the cleavage of C–C, C–O, C–N, and other bonds by eliminating atoms, leaving double bonds.
- Oxidoreductase: catalyzes oxidation and reduction.
- Hydrolases: These will catalyze and lead to hydrolytic cleavage of C–C, C–O, C–N, and other bonds.
- Ligases: These will catalyze the joining of two molecules coupled to the hydrolysis of ATP.
- Isomerases: These will catalyze geometric or structural changes within a molecule.
What enzymes are found in the blood?
- Cellular enzymes are found in cells and are secreted into the blood when cells are injured, such as LDH, alkaline phosphatase, aminotransferases, and others.
- Secreted enzymes like lipase, amylase, trypsin, and acid phosphatase are secreted from the glands.
- Plasma-specific enzymes are serine proteases, thrombin, factor X, and plasminogen.
- Functional plasma enzymes:
- Certain enzymes, proenzymes, and their substrates are present all the time in the blood of normal individuals.
- These enzymes perform normal physiologic functions.
- Examples are lipoprotein lipase, pseudocholinesterase, and the proenzyme of the coagulation process.
- Mostly, these enzymes are secreted and synthesized in the liver.
- Nonfunctional plasma enzymes:
- These are physiologic enzymes in the blood with no known physiologic function.
- These are liberated from the ruptured RBCs, WBCs, and other cells.
- These enzyme levels increase in response to cell injury.
What are the variables for the functional activity of enzymes?
- Factors affecting the rate of enzyme catalytic reaction depend upon the following:
- The concentration of the enzymes:
- The enzyme’s role depends on its concentration.
- The reaction velocity is directly proportional to the enzyme concentration.
- The reaction is more rapid when there is an adequate amount of enzyme to bind the abundant substrate; this is a mass-action effect.
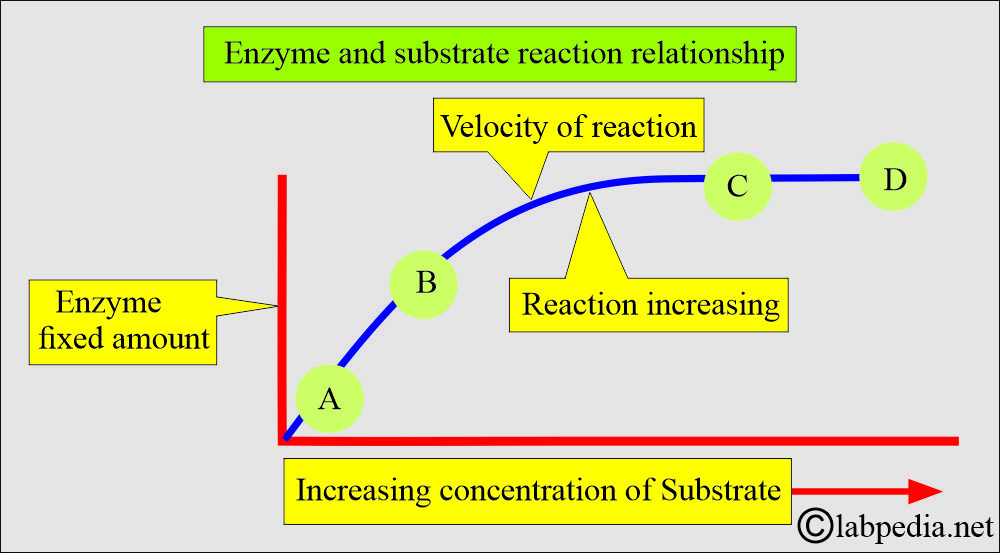
- The concentration of the substrate:
- It depends upon the concentration of the substrate.
- A curve is shown in the following diagram when a fixed amount of enzyme is used against increasing amounts of substrate.
- With a fixed enzyme and increasing substrate concentration, the reaction reaches a plateau; there is no change after that.
- After points C and D, the reaction reached the plateau.
- The reaction will fall to zero.
- pH:
- The enzymes are proteins that carry a negative charge.
- These negatively charged molecules are pH-sensitive.
- The binding of the enzyme with the substrate is pH-dependent.
- pH plays a role in the enzyme reaction. Most enzymes react between a pH of 7 to 8.
- Few react at low pH, like pepsin at 1.5.
- While alkaline phosphatase reacts at a pH of 10.5
- An optimum pH is needed for various reactions.
- Temperature:
- Enzyme reaction depends upon the temperature.
- The rate of the enzyme reaction velocity is proportional to the temperature.
- The reaction velocity doubles for every 10 °C rise in temperature.
- Enzymes are proteins, so they are very sensitive to temperature.
- When enzymes are exposed to temperatures of 60 °C and above, they are mostly denatured.
- Mostly, our enzymes are effective at 37 °C to 38 °C. Their activity decreases when the temperature is above 42 °C to 45 °C.
- Enzyme activity is preserved when stored at low temperatures. Enzyme activity can be maintained for months at temperatures ranging from -20 °C to -70 °C.
- Ionic strength:
- Most enzymes are sensitive to some of the buffer ions in the incubation mixture.
- Some cations at the appropriate concentration are needed for optimal enzyme activity.
- Presence of specific ions like an activator or an inhibitor.
- Any other substance that alters the enzyme configuration affects its activity.
What are the inhibitors of the enzyme reaction?
- These inhibitors may be either reversible or irreversible.
- Reversible inhibitors:
- In this case, enzyme activity returns to normal when the inhibitor is removed from the body.
- Irreversible inhibitors:
- These inhibitors bind covalently to the enzyme. Their effect progresses over time; it is complete when their amount exceeds that of the enzyme.
- Inhibition by the antibody:
- Sometimes there is no effect of the antibody, but in other cases it may reduce or abolish enzyme activity.
- Enzyme names are in two parts:
- The first part is from the substrate.
- The second part ends with “case.”
What are the functions and characteristics of enzymes?
- Enzymes do not affect the equilibrium point.
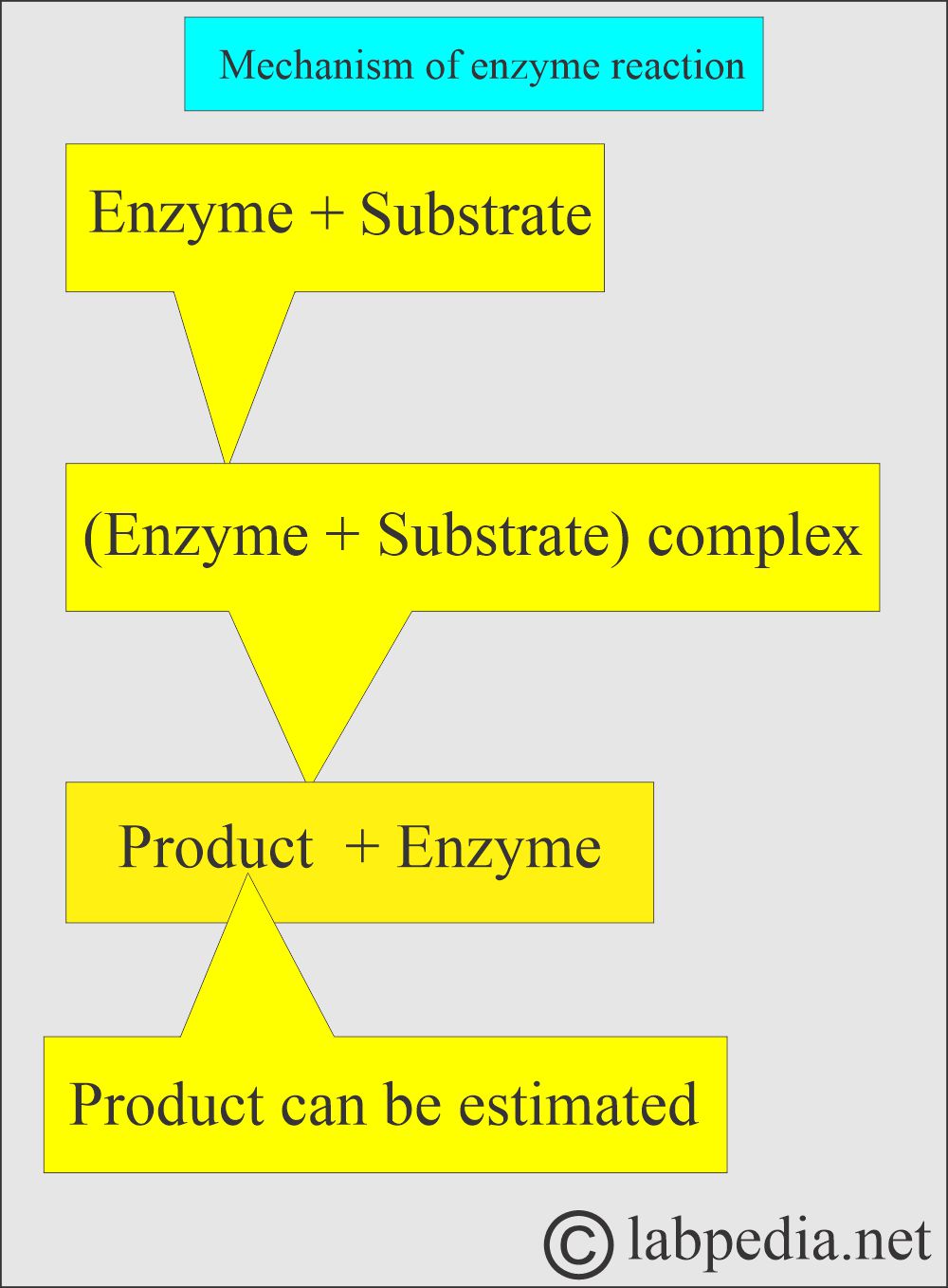
- Enzymes enter into the reaction but are not consumed.
- Enzymes bind to their substrates and form enzyme-substrate complexes.
- Enzyme + Substrate = Product + Enzyme.
- Enzymes are present at very low concentrations, so they are usually not measured; instead, they are assessed by what they do.
- So basically, to assess the enzymes, you look at the decrease in substrate or the increase in product.
- The enzyme increases:
- By increasing leakage from the cells.
- This is seen in cell stress or cell necrosis.
- Increased production of the enzyme.
- Some drugs increase enzyme production.
- Cell proliferation produces specific enzymes.
- By increasing leakage from the cells.
What are the factors affecting enzyme levels?
- Hemolysis:
- There is increased activity in the LDH because of increased LDH in the RBCs (150 times more than the serum). So, traces of hemolysis will increase the level.
- SGOT (AST) is also elevated due to this enzyme in the RBCs. (There is 15 times more activity in the RBCs).
- Acid phosphatase is mildly raised due to hemolysis.
- There is increased activity in the LDH because of increased LDH in the RBCs (150 times more than the serum). So, traces of hemolysis will increase the level.
- SGOT (AST) is also elevated due to this enzyme in the RBCs. (There are 15 times more active in the RBCs).
- Acid phosphatase is mildly raised due to hemolysis.
- Effect of storage:
- Serum or plasma should be separated from RBCs as soon as possible.
- Enzymes are usually stable for 8 to 12 hours at room temperature in the serum or plasma.
- While enzymes are stable for 5 to 7 days at -4 °C.
- When kept at -20 °C, it is stable for weeks to months.
- At -70° C, these are stable for an indefinite period.
- Patient variables:
- A fasting sample is always preferred.
- The alkaline phosphatase level is elevated after eating the food (the Intestine has a high alkaline phosphatase level).
- The alkaline phosphatase level is very high at birth and in growing children. It comes to the adult level by 15 to 17 years in females and 18 to 20 years in males. Overall, there is a variation of these enzymes with age.
- SGOT (AST) and SGPT (ALT) are raised at birth and reach a normal level by 2 to 4 months.
- Gamma GT (glutamyltransferase) is elevated at birth and reaches adult levels by 6 months of age.
- Aldolase is also raised at birth and in young children. It reaches a normal level by the age of 16.
- Creatinine kinase is higher in males than in females.
- AST, Creatine kinase, and LDH may be elevated after exercise.
- Intramuscular injection may increase the value of Creatinine Kinase, AST, and LDH.
- Leakage of the enzyme from the cells.
- Altered enzyme production.
- Clearance of the enzyme.
What are the clinically important enzymes commonly used in various diseases?
| Enzymes | Source of these enzymes | Diagnostic importance |
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
How will you summarize the enzymes?
- Enzymes are highly effective catalysts in biochemical reactions and are extremely specific.
- The catalytic activity of the enzymes shows:
- Their presence.
- Help in their detection.
- Provides the basis for an enzyme-linked immunoassay.
- Assay of the plasma enzymes helps in:
- Diagnosis of diseases like myocardial infarction and viral hepatitis.
- Help to evaluate prognosis.
- Most enzymes are assayed spectrophotometrically.
- Enzymes are present in all body cells, each functioning in a specific reaction.
- Enzymes catalyze all essential reactions, such as oxidation, reduction, hydrolysis, esterification, and synthesis, that provide the energy and chemical changes necessary for vital activities.
- Enzymes help in:
- Nerve conduction.
- Muscle contraction.
- Digestion.
- Respiration.
- Maintenance of body temperature.
- Reproduction.
- Growth.
- Digestion.
- Enzymes are controlled by genes.
Questions and answers:
Question 1: Are enzymes consumed in the chemical reaction?
Question 2: What is the importance of CK-MB?