January 24, 2026
Diagnostic Value of Enzymes in Various Diseases
Creatine kinase (CK)
How will you define Creatine kinase (CK)?
- This is a dimeric enzyme that causes the reversible phosphorylation of creatine.
- CK activity is greatest in striated muscles, the brain, and the heart tissues.
- The kidney has lower lipase activity.
- The liver and RBCs have no CK activity.
What are the indications for creatine kinase (CK)?
- Skeletal muscle disorders.
- Cardiac muscular diseases.
- Neuromuscular diseases.
- Drug and toxin-induced diseases.
- Metabolic and endocrine conditions.
What are the subunits of creatine kinase?
- CK-1 is CK-BB.
- CK-2 is CK- MB.
- CK-3 is CK – MM.
What are the conditions where creatinine kinase increases?
- CK enzyme activity increases in skeletal muscles, the cardiovascular system, the brain, and thyroid diseases.
- CK-MM is increased in:
- Muscular atrophy, especially Duchenne’s type.
- In progressive muscular dystrophy.
- The enzyme level is high in children between 7 to 10 years.
- It falls in the older patients.
- CK-MB is increased in:
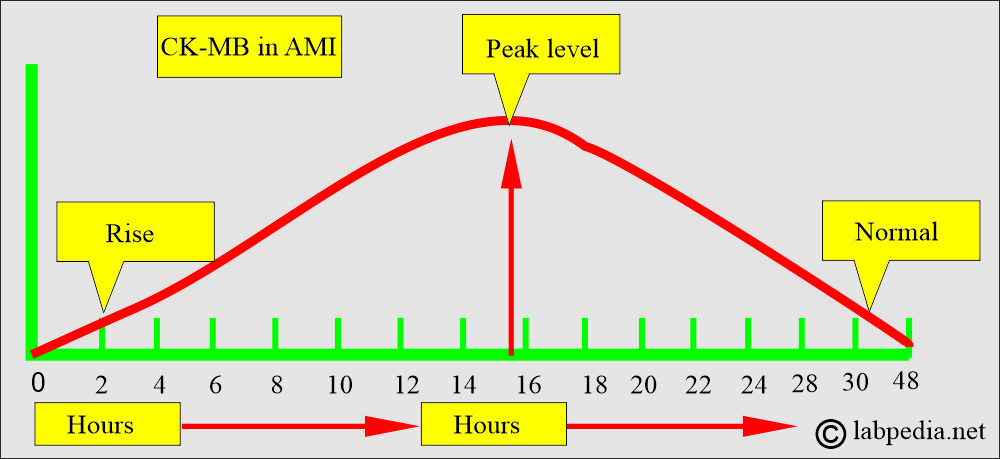
- It is raised after an acute myocardial infection.
- CK-BB is increased in :
- It is increased in cerebrovascular diseases.
- It increases in the neurosurgical intervention.
- In cerebral ischemia.
Gamma-glutamyl transferase (GGT)
How will you define Gamma-Glutamyl Transferase (GGT)?
- GGT is a membrane-bound enzyme in the liver (cells lining the bile ductuli and canaliculi).
- GGT is also seen in proximal renal tubules, the brain, the prostate, and the pancreas (ductules and acinar cells).
What are the Indications for the GGT:
- It is advised for liver diseases.
- It is advised to limit alcohol intake to prevent liver cell injury.
- It differentiates liver diseases from bone diseases, where alkaline phosphatase is raised.
How will you explain the pathophysiology of GGT?
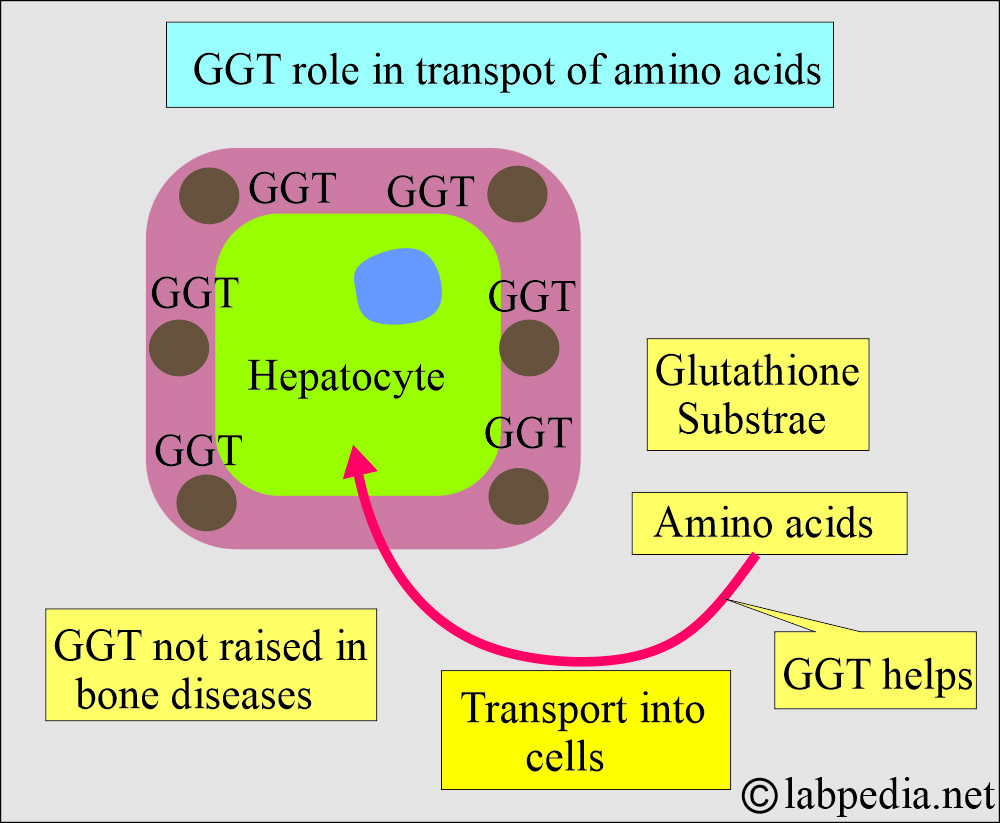
- GGT is responsible for glutathione’s extracellular metabolism (the cells’ main oxidant).
- Glutathione is its substrate.
- GGT is present on cell membranes and assists in the transport of amino acids into cells.
- GGT is mainly present in the liver cells. It is also presented in the biliary tract epithelium.
- GGT is present in the smooth endoplasmic reticulum of liver cells, and it will be increased in case of increased toxins.
- GGT is increased in patients when exposed to drugs like barbiturates, warfarin, valproate, Dilantin, methotrexate, and alcohol.
- A small amount is found in the kidneys, intestine, heart, brain, pancreas, and spleen.
- There is some activity in the capillary endothelial cells.
- The serum GGT level increases in the newborn but decreases to the adult level by 4 months.
- GGT usually does not increase in bone disease, childhood or adolescence, or pregnancy.
- GGT is raised by acute liver cell damage and biliary tract obstruction.
- Its half-life is 7 to 10 days.
- This enzyme originates from the hepatobiliary system.
- GGT is the best indicator of occult alcoholism.
- GGT is lower in women than in men. It is significantly higher in African Americans.
What are the Causes of raised GGT?
- It is raised 2 to 3 times the normal value in heavy drinkers.
- It returns to normal after stopping the alcohol in about 3 weeks.
- It is raised in all forms of liver disease.
- It is raised in intrahepatic and posthepatic biliary obstruction.
- The level reaches 5 to 30 times the normal value.
- This is a more sensitive test for diagnosing obstructive jaundice, cholangitis, and cholecystitis.
- The rise is earlier than that of other enzymes and persists longer.
- Moderate elevation, 2 to 5 times, occurs in infectious hepatitis.
- A high level was seen in primary or metastatic malignancies.
- A 2 to 5 times increase is seen in fatty liver and drug intoxication.
- In acute or chronic pancreatitis, GGT levels may be elevated to 5-15 times normal when a hepatobiliary obstruction occurs.
- GGT is always raised in acute pancreatitis. In chronic pancreatitis, it is raised if there is biliary tract involvement or active inflammation.
- It is normal in a skeletal disease like Paget’s disease and in bone malignancy, even when alkaline phosphatase is raised.
- GGT is raised in alcoholic cirrhosis.
- In 5% to 30% of patients, GGT is raised in acute myocardial infarction. This is due to the proliferation of capillaries and fibroblasts in granulation tissue. The rise is usually reported after 7 to 14 days of the infarction. Usually, elevation starts on the 4th to 5th day and peaks at 8 to 12 days.
- In pregnancy, in bone diseases, and in childhood, alkaline phosphatase levels increase, but GGT remains normal.
What is the importance of GGT?
- GGT differentiates from the liver to non-liver cell injury origin when the alkaline phosphatase level is raised.
- GGT is the best test to confirm that elevated alkaline phosphatase is of hepatic origin.
Lactate dehydrogenase (LDH)
What sample for LDH is needed?
- It is done in the patient’s serum.
- A random sample can be used.
What are the Indications for LDH?
- It is a marker of hemolysis.
- It is a useful marker of the disease activity in cryptogenic fibrosing alveolitis and extrinsic allergic alveolitis.
- LDH was the marker of AMI, but is now replaced by Troponin-T.
What are the precautions for LDH?
- Avoid hemolysis, as this will interfere with LDH estimation.
- Separate the clot from the serum immediately.
- Avoid heating the blood sample.
How will you explain the distribution of LDH?
- LDH is widely distributed in tissues and has a high concentration in the liver, cardiac muscle, kidneys, skeletal muscle, RBCs, and other tissues.
- Less concentration is found in the lungs, smooth muscles, and the brain.
What are the isoenzymes of LDH?
- LD-1 is predominant in the heart, muscles, kidneys, and RBCs.
- LD-2 is like LD-1.
- LD-3 is present in various tissues, such as the lung and spleen.
- LD-4 is more prevalent in the liver and skeletal muscles.
- LD-5 is more concentrated in the liver and skeletal muscles.
- LDH- 5 has isoenzymes:
| LDH isoenzyme | % of isoenzyme | Clinical use and source |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What is the chemical role of LDH?
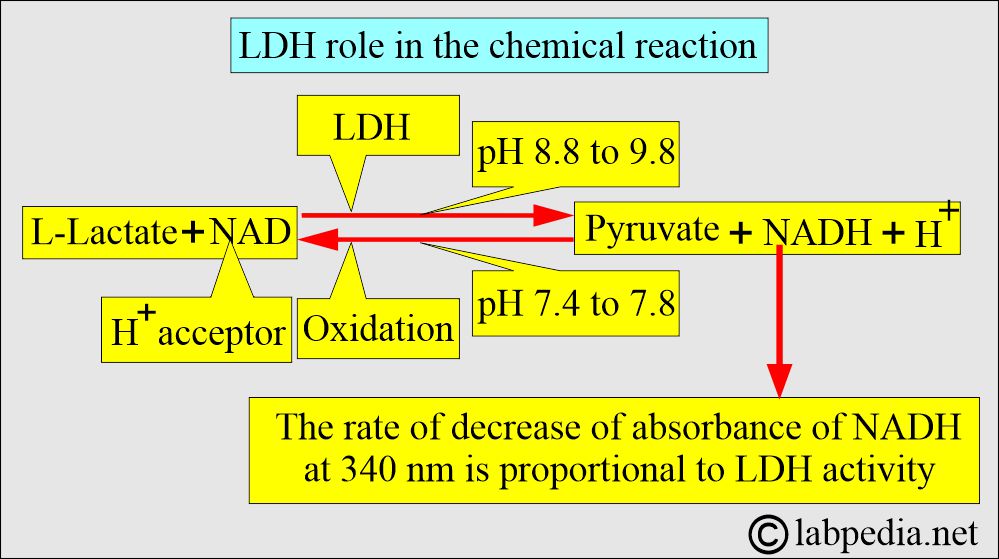
- LDH catalyzes the interconversion of lactate to pyruvate.
- Lactate dehydrogenase is a hydrogen transfer enzyme that catalyzes the oxidation of L-lactate to pyruvate.
- The optimal pH for LDH-catalyzed pyruvate formation is 8.8-9.8.
- LDH activity is present in all cells and is found in the cytoplasm.
- Enzyme levels vary across various tissues.
- The tissue level is 500 times higher than the serum, so cell leakage increases the serum level.
What are the causes of increased LDH levels?
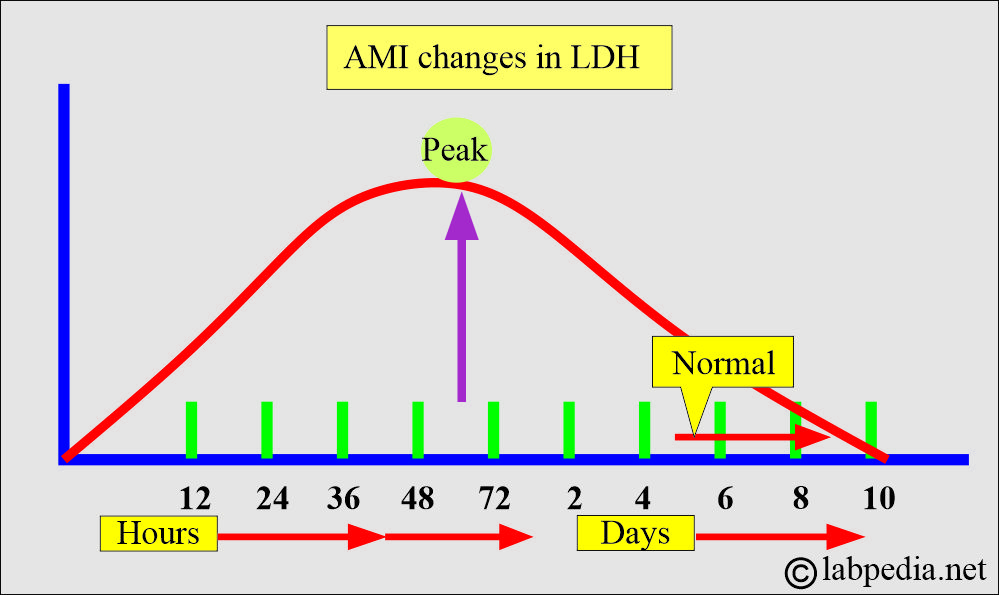
- Acute myocardial infarction:
- LDH levels rise within 24 to 48 hours of the AMI, peak at 48 to 72 hours, and slowly return to normal over 5 to 10 days.
- A moderate increase may be seen in myocarditis and cardiac failure.
- Severe shock and anoxia.
- Megaloblastic anemia.
- In the case of liver disease.
- In 1/3 of cases of renal disease, such as tubular necrosis and pyelonephritis.
- In liver metastatic tumor infiltration.
- LDH-raised values in the urine are seen in glomerulonephritis, diabetic nephropathy, systemic lupus erythematosus, urinary bladder, and kidney malignancies.
- CSF infiltration by granulocytes increases LDH levels.
- In bacterial meningitis, LD-4 and LD-5 increase.
- In cerebrovascular accidents:
- Subdural and subarachnoid hemorrhage increases all isoenzymes of LD, especially LD-3, 4, 5
- Where the peak level is reached in 1 to 3 days.
- It is not related to xanthochromia.
- In CNS tumors, there is an increase in the LD-5 in >9% of the cases and a decreased level of the LD-1: LD-5 ratio.
- LD-1: LD-5 ratio <2.5 in the absence of infection or hemorrhage suggests meningeal tumors.
- LD-5 in >10% of the cases suggests high-grade malignant tumors.
- This is normal in angina and pericarditis.
Lipase Enzyme
How will you define lipase?
- Lipase is a glycoprotein enzyme filtered by glomeruli and completely absorbed by proximal tubules.
- Lipase is more specific than amylase in pancreatic damage.
How will you discuss the distribution of the lipase enzyme?
- The pancreas is the main organ for lipase secretion, which goes into pancreatic juice.
- Lipase is found in the stomach and intestinal mucosa, but it doesn’t play a significant role in fat processing.
- Pancreatic lipase is secreted into the duodenum, where it converts triglycerides into fatty acids.
- Lipase concentration in the pancreas is 100 times greater than in other tissues.
- The difference between amylase in the pancreas and serum is 20,000 times.
- A very small amount is found in the serum.
- Lipase estimation is difficult and not reproducible, so it is not common in labs.
- Lipase hydrolyzes glycerol esters of long-chain fatty acids.
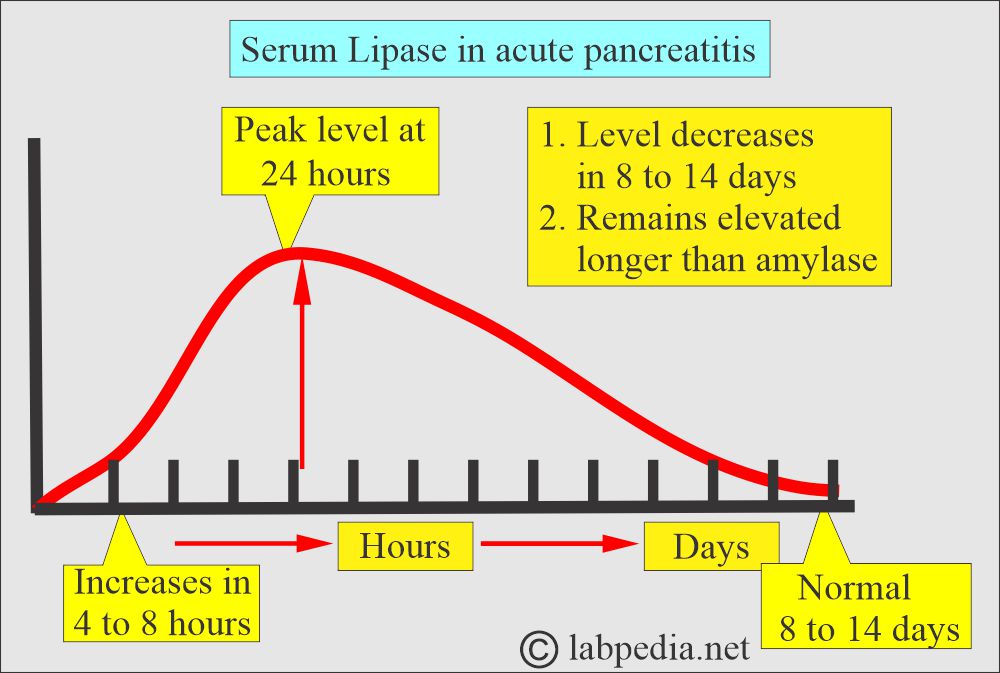
How will you discuss Acute pancreatitis?
- Lipase enzymes can diagnose it.
- The serum lipase level rises slightly later than the amylase.
- The initial rise is seen between 4 and 8 (3 and 6) hours.
- The peak level is 24 hours (another reference says it occurs after 72 to 96 hours).
- The peak remains for a longer time.
- The normal level is seen after 8 to 14 days (7 to 10 days).
What are the conditions under which the Lipase level is raised?
- Acute pancreatitis.
- Chronic pancreatitis.
- Acute cholecystitis.
- Perforating or penetrating peptic ulcer.
- Obstruction of the pancreatic duct by:
- Stones.
- Drugs induced.
- Partial obstruction with the use of drugs.
- Small intestinal obstruction.
- Intestinal infarction.
- Acute and chronic renal failure with complications.
- Alcoholism.
- Diabetic ketoacidosis.
- Drug-induced acute pancreatitis.
What are the conditions under which the Lipase level decreases?
- It is only observed in the methodology, where interference from Hemoglobin, heavy metals, and calcium ions is present.
What are the conditions where the Lipase level is normal?
- Values are lower in neonates.
- Mumps.
- Macroamylasemia.
Questions and answers:
Question 1: Which one is more specific for acute pancreatitis, amylase or lipase?
Question 2: What is the value of LDH in angina and cardiac failure?