Chapter 9: Complement (Complement System)
COMPLEMENT
Complements are essential proteins. These are called complement because it complements the antibacterial activity of some of the antibodies. Compliments are given numerical names as C1 to C9, which are 20 including subunits. Their concentration is 3-4 G/L in the blood.
Complement makes 10% of the serum proteins.
Functions
- Activation of complements leads to inflammation and localize the antigen or cause lysis.
- Once complement is activated, its components participate in virtually every aspect of the inflammatory response.
- There is an antimicrobial activity.
- Serum sickness-like immune reaction.
- Autoimmune diseases.
- These are not increased by infection or other antigens.
- But IL-1 and γ-interferon increase the synthesis.
Site of Action
The main site is cell surface or other biological membranes.
Changes by Activation of Complement
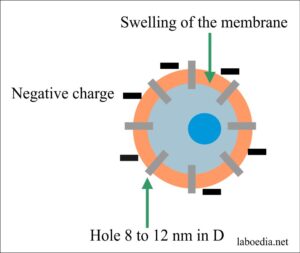
- There are ultrastructural changes in the cell membrane.
- There are changes in the electrical charges of the cell membrane.
- There is swelling of the membrane.
- Ultimately there are circular holes 8-12 nm in diameter.
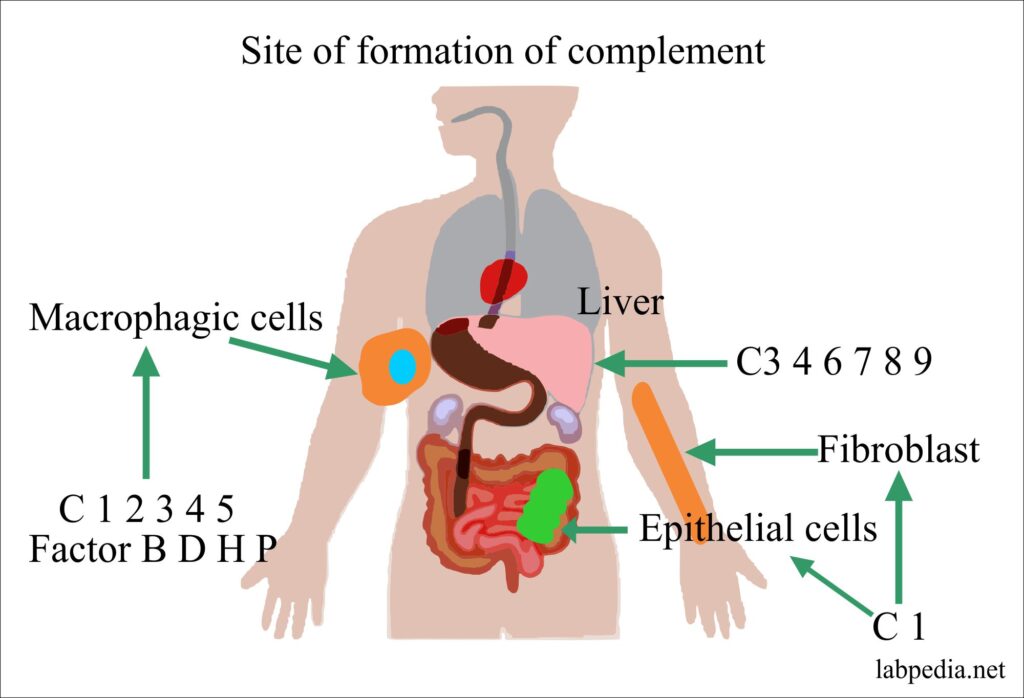
Site of Formation
- The complement may be synthesized in the intestinal epithelium, macrophagic cells, and spleen.
- C1 is a calcium dependant complex and consists of C1q, C1r, and C1s, this is synthesized in the epithelium of the gastrointestinal and urogenital epithelium
| Complement | Site of Synthesis |
| C1 | Synthesized in Epithelial cells and fibroblasts. |
| C3 4 6 7 8 9 | The liver is the major site of formation. |
| C1 2 3 4 5 | Synthesized by macrophagic cells. |
| Factor B, D, H, P | Synthesized by macrophagic cells. |
Table X – Site of Complement Formation
Fetus: Starts forming complement by the second month of pregnancy.
Complement Activation
Complement activation consists of:
- Recognition of antigen and antibody.
- Enzymatic chain reaction.
- Formation of membrane attacking complex which leads to cellular destruction
After Activation Complement is cleaved into:
- Complements are present in an inactive form in blood circulation, once activated they give chain reaction like blood coagulation factors.
- Larger molecule labeled as “b”. It leads to further activation of the chain reaction.
- A smaller molecule labeled as “a” it promotes inflammation and produces pharmacological action.
- Further proteolysis gives inactive component labeled as “iC3 b”.
- Complement is catabolized in the body 1-3% per hour.
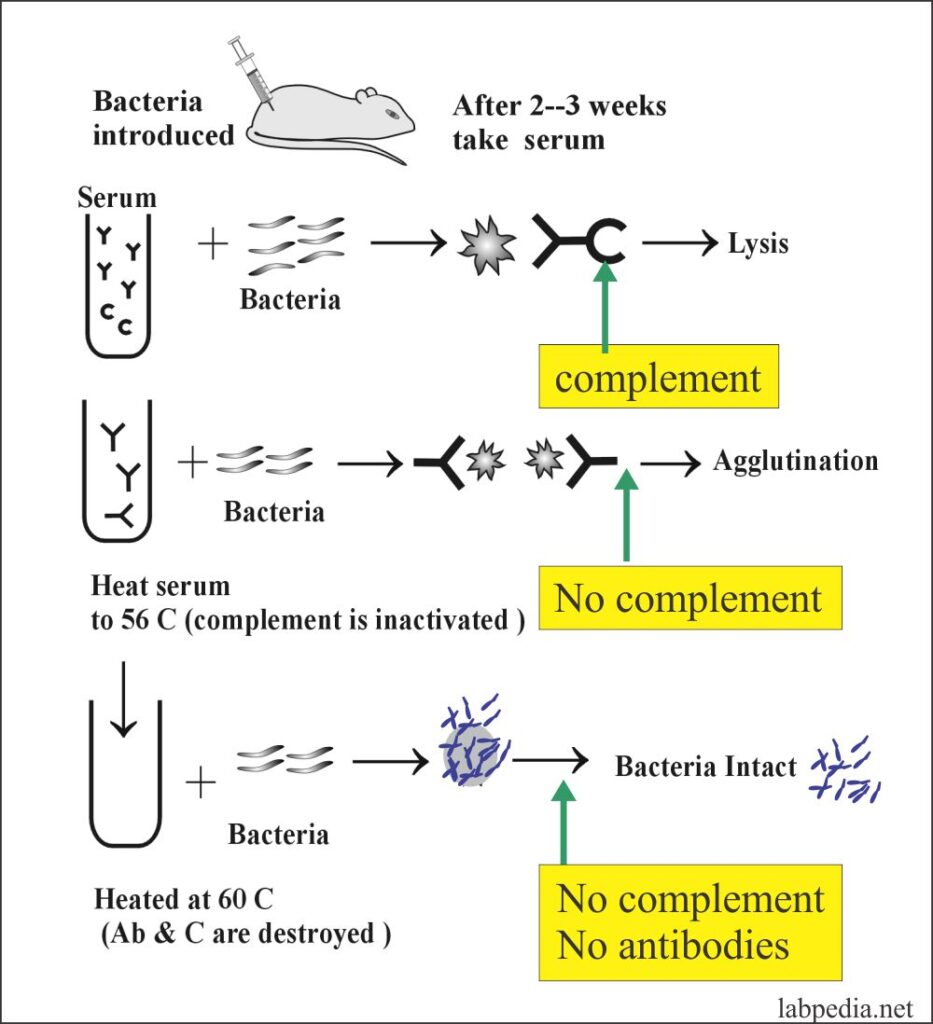
The following example shows the role of Antibodies and complement in the process of Bacteriolysis and Agglutination.
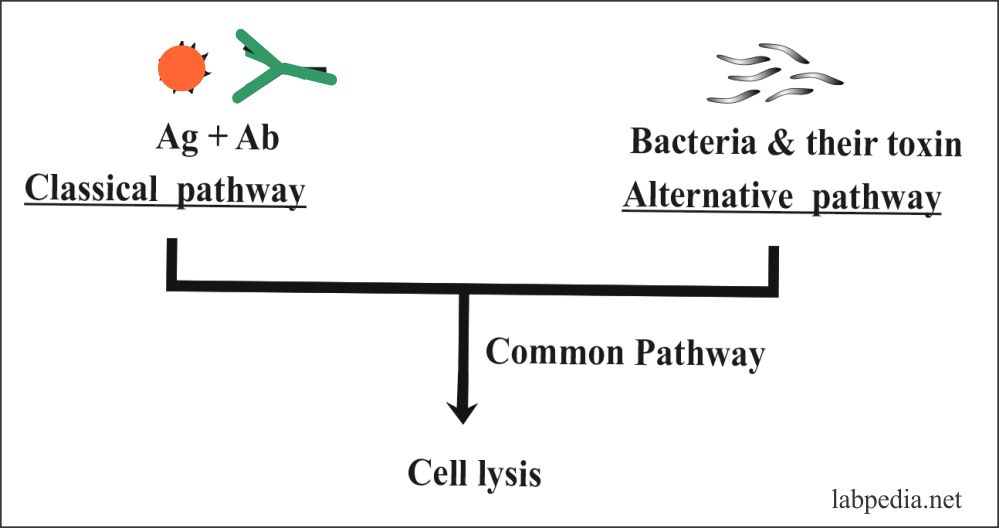
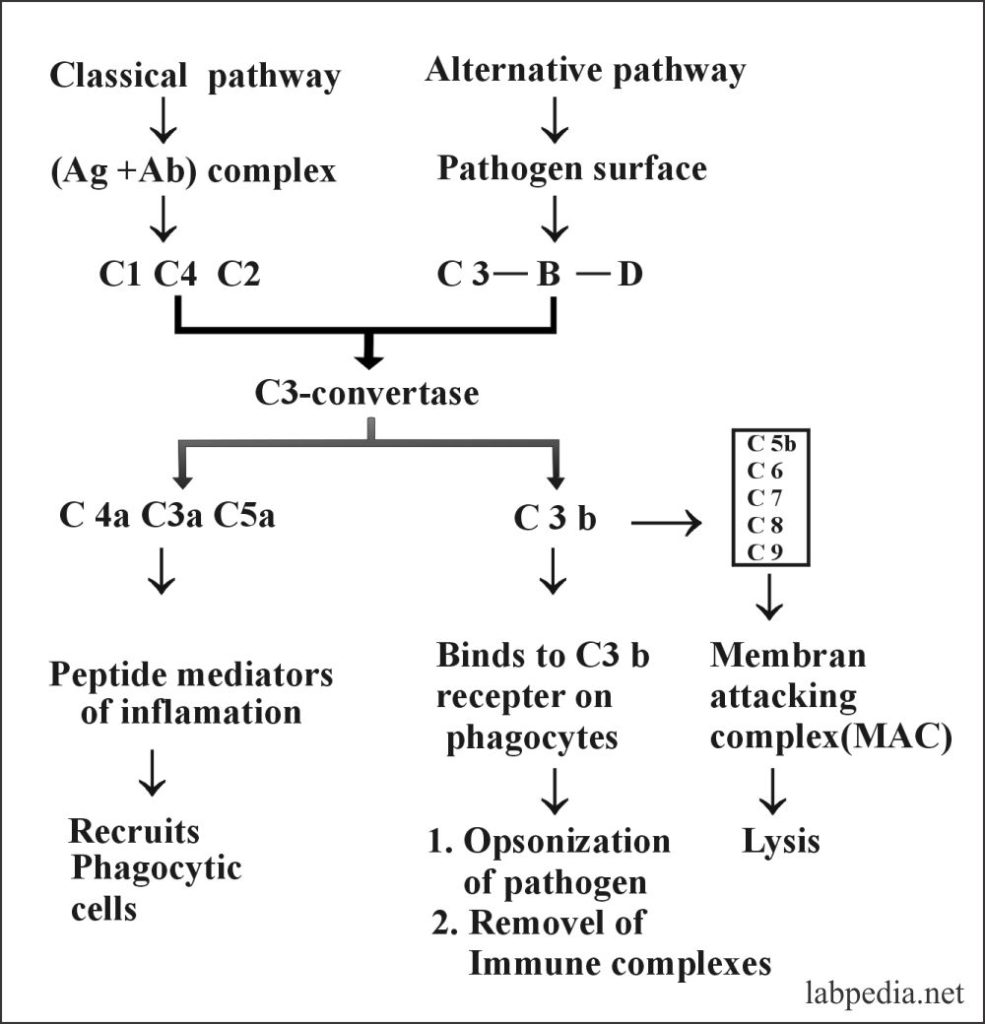
Complement pathways
- Classical pathway
- Alternative pathway
The classical pathway may be activated by:
- Immunological stimuli-like Ag & Ab complex, tissue injury, or aggregated IgG.
- non-immunological stimuli like CRP, DNA, Trypsin, E. Coli, Salmonella, viruses, endotoxin, and urate crystals.
Alternative Pathway may be activated by:
- Immunological stimuli like aggregated IgA, sometimes IgG.
- Non- immunological stimuli are lipopolysaccharides (endotoxin), Trypsin & trypsin-like enzyme, cobra venom. Parasite & teichoic acid of gram-positive bacteria.
The following diagram gives a summary of the Classical pathway and Alternative pathway.
Activation of Complement leads to:
- Lysis of Bacteria, viruses, and cells.
- Mediate acute inflammation.
- This leads to the release of histamine.
- It helps in opsonization and phagocytosis.
- It has a regulatory role and it regulates acute inflammation in the immune response.
Various Complement-Proteins lead to:
- Vasodilatation at the site of inflammation.
- Increase adherence of phagocytic cells to blood vessels endothelium.
- It directs movements of phagocytic cells to the area of inflammation.
- Ultimately clears of the infection.
The outcome of Complement Activation:
- Physiologic action:
- Vasodilatation at the site of inflammation.
- Increased vascular permeability.
- Cellular action:
- Increase adherence of phagocytic cells to blood vessels endothelium.
- Recruitment of acute inflammatory cells.
- It directs movements of phagocytic cells to the area of inflammation.
- Produce inflammatory mediators.
- Hemolysis in the case of RBCs.
- Cytolysis.
- Opsonization of the pathogens.
- Ultimately clears of the infection.
- Ultimately killing of pathogens.
Complement Receptors
Various cell types express surface membrane glycoproteins that react with one or more of the fragments of C3 produced during complement activation and degradation.
Complement receptor 1 (CR1) is important in increasing phagocytosis. CR1 is an important factor present on the RBCs.
| Type of receptor | CD molecule |
Specificity for the receptor | Distribution on the cells | Main functions |
| CR1 (Complement receptor 1) | CD35 | C3b |
|
|
| CR2 (Complete receptor 2) | CD21 | C3d, |
|
|
| CR3 (Macrophage-1-antigen) | CD11b, CD18 | iC3b |
|
|
CLASSICAL PATHWAY ACTIVATION
Overview
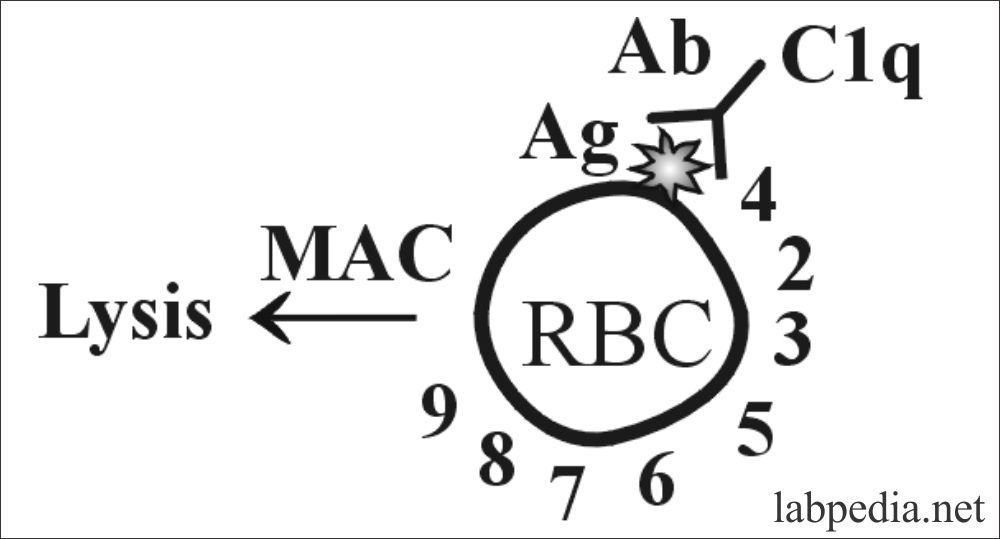
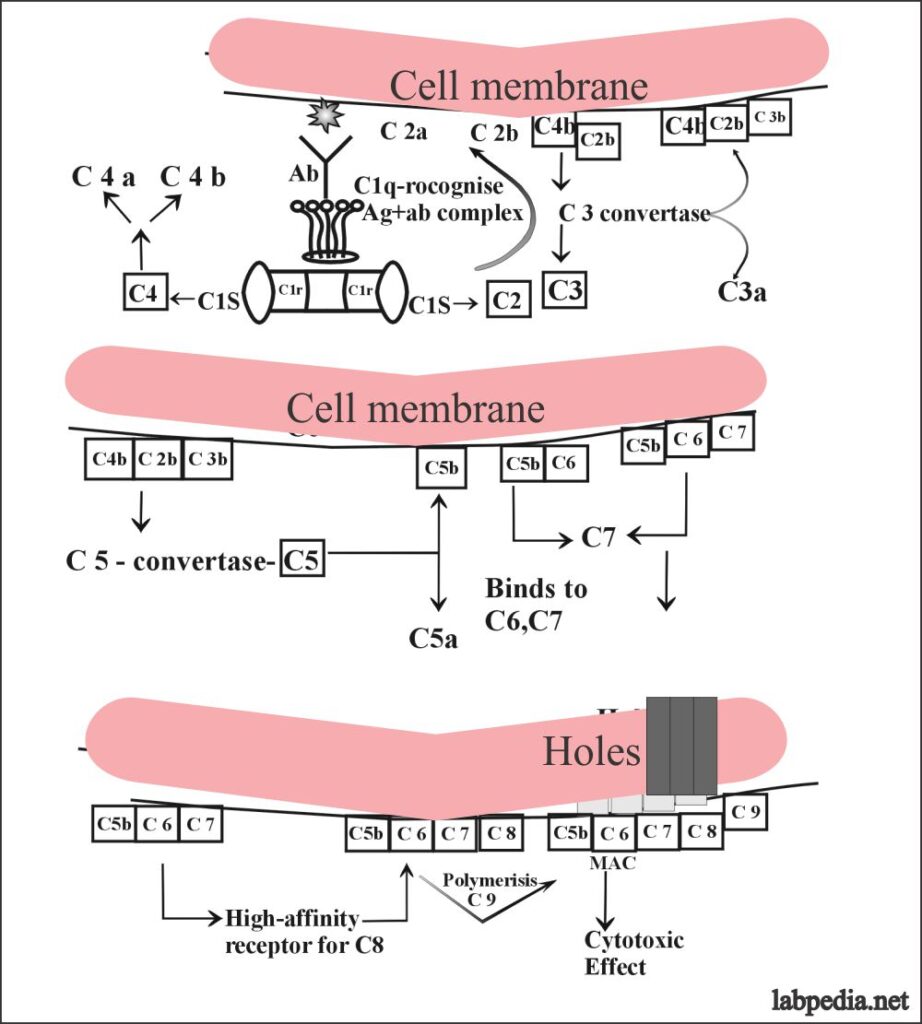
Suppose this reaction is taking place on the cell membrane of the RBC and the sequence of the event may be as follows:
This can also be explained in the following diagram in a more simple way.
In the classic pathway antigen and antibody complex is recognized by the complement (C1q) and it starts a chain reaction by stimulating C1s and C1r which will stimulate C4 and C2. Then there is the stimulation of C3 C5 C6, 7, and ultimately C9.
This will give rise to MAC (membrane attacking complex) and leads to the osmotic death of the target cells.
Details of Classical Pathway Activation
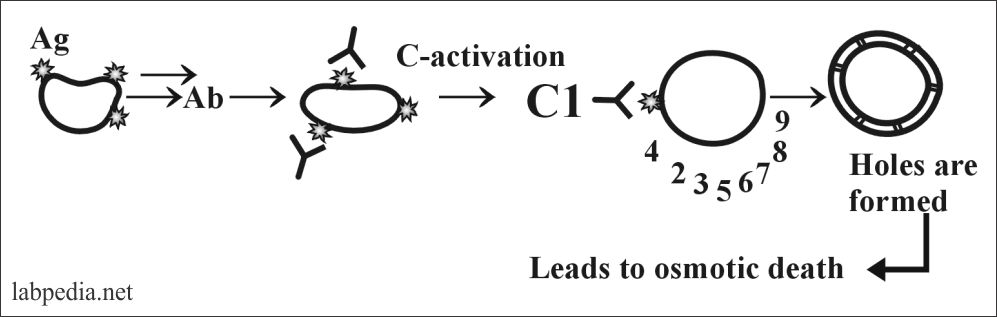
Now the details of the classical pathway can be elaborated when suppose the reaction is occurring on the cell membrane where there is an antigenic determinant (Epitope).
The activation of complement is not in sequence from C1 to C9, but this is like this:
C1, 4, 2, 3, 5, 6, 7, 8 and 9.
The sequence of activation of the classical complement pathway:
- C1q is the recognition unite and it recognizes the Ag-Ab complex and then activates C1r.
-
- C1q directly binds to the immunoglobulin molecule.
- C1r and C1s do not bind the immunoglobulin but leads to the subsequent activation of C3.
- C1q only attaches to subclasses of IgG and IgM.
- IgM single molecule is able to fic C1 but IgG needs two molecules.
- C1q is functionally multivalent for to attach to the complement fixation sites of immunoglobulin.
- C1r and C1s are similar in structure, where C1r forms dimers and C1s binds to C1r.
- Now, C1s and C1q both bind to C1q in the presence of Ca++.
- C1s is the only substrate for C1r.
- C1r– cleaves C1S (C1S–).
- C1s cleaves C4 to C4b (large components) and C4a.
- This function takes place in the fluid phase of the plasma around the C1s.
- C1s are weekly proteolytic for free C2 but highly active against C2 that has complexed with C4b molecule in the presence of Mg++ ions.
- C1s also cleaves C2 to C2a and C2b.
- This reaction takes place when the C4bC2 complex is near the C1s.
- The control mechanism for the activity of C1s is a C1-proteinase inhibitor, which will control the C1s for un-controlled activation of C4 and C2.
- C3b esterase inhibitor has the ability to disintegrate the membrane-bound C4b.
- This action destroys the acceptor site for C2, and this will prevent the formation of the C4bC2b convertase complex.
-
- C4b molecule has a stable binding to the cell membrane (efficiency is <10%).
- C4b molecule which can not attach to the cell membrane will become inactive and decay.
- The resulting C2b joins the C4b and forms the complex C4bC2b which is an enzyme.
- C4bC2b complex enzyme is unstable and decays with a half-life of 5 minutes at 37 °C, due to the release and decay of C2b.
- C4b and C2b act as C3-convertase and cleaves C3 to C3a and C3b.
- C4bC2b convertase will activate the C3 molecule into C3a anaphylatoxin and C3b.
- C3b: Clusters of C3b are activated and bound near the C4bC2b complex.
- Each catalytic site can bind several hundreds of C3b molecules.
- Only a C3b molecule combines with C4bC2b to form the final proteolytic complex complement pathway.
- The complex of C4bC2bC3b forms an opsonic macromolecular coat on the target cells and makes it susceptible to immune adherence by the C3b receptor on the phagocytic cells.
- C4b C2b C3b acts as a C5 convertase and cleaves C5 into C5a and C5b.
- C5b fixation and is the beginning of the membrane attack complex.
- No further proteinases are generated in the classical complement pathway.
- C5b binds to C6 and make a labile reactive site for C7.
- C5b 6, 7 is highly lipophilic and binds to the membrane where it acts high-affinity receptors for C8.
- In free solution, uncombined C567 has a half-life of about 0.1 seconds.
- It can attach to any lipid layers within its effective diffusion radius and will produce the picture of reactive lysis on innocent bystanders cells.
- Membrane-bound C567 is stable and can interact with C8 and C9.
- C8 has three chains α, β, and γ where γ-chains insert into the membrane.
- C5b678 polymerizes C9 forming tubule the MAC (membrane attacking complex), which gives rise to hole formation in the cell-membrane Like drill machine.
- This tubule is a hollow cylinder with one end inserted into the lipid layer and other ends can project from the membrane.
- C5b678 makes a 3nm hole and cells become leaky.
- C5b6789 makes transmembrane holes, 15-16nm long and 8-12nm in diameter. This leads to the passage of electrolytes and water and causes osmotic lysis (death).
- By combing with C9, the cytolytic reaction is accelerated.
- This hole formation can disturb the lipid layer and allow the free exchange of ions as well as water across the membrane.
- The end result is in the living cell is an influx of Na+ and H2O leads to disruption of the osmotic balance which produces cell lysis.
Functions of Various Components:
- C1q
- It is evaluated in the serum.
- It is needed in the first reaction of complement where it will lead to activation of C4 and C2.
- C2 and C2a
- It takes part in the activation of C3 with the help of C4.
- It increases vascular permeability.
- It causes contraction of smooth muscles.
- Some say that functions are unknown.
- C4a
- It is a weak anaphylatoxin and weak mediator of Inflammation.
- C3a
- It is a strong anaphylatoxin.
- It leads to an increase in vascular permeability and smooth muscle contraction.
- This leads to the release of vasoactive amines.
- Leads to the release of lysosomal enzyme.
- It is chemotactic.
- C5a
- Functions just like C3a.
- It increases neutrophil activity.
- More potent chemotactic factor than C3a.
- C6
- Activated by the C5b and attach to C7.
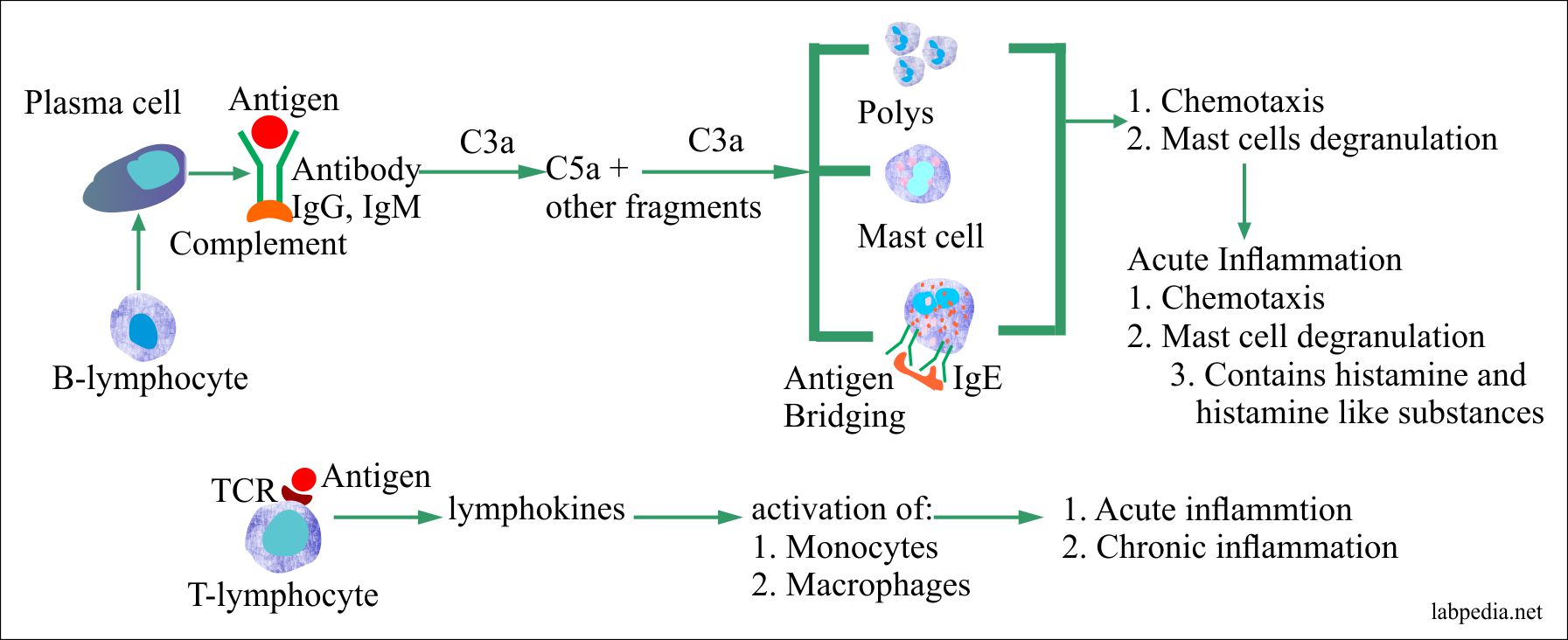
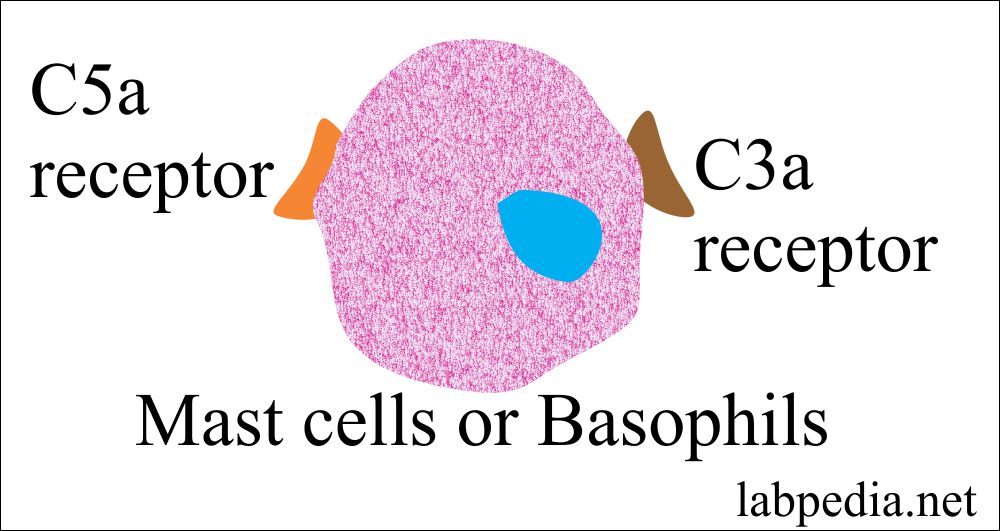
Anaphylatoxin
- These are C3a, C5a, and C4a.
- This name is because of their role in anaphylaxis, where these directly activate mast cells and basophils, through their receptor for C5a, and lesser extent C3a.
- The anaphylatoxin activity is:
- Smooth muscle contraction.
- Vasodilatation.
- Increases vascular permeability.
- Give rise to the release of histamine.
- These are chemotactic agents.
- These initiate the inflammation.
- These can generate cytotoxic oxygen radicals.
- Their main function is degranulation of the mast cells or basophils, endothelial cells, and phagocytes.
- C5a has the highest biologic activity.
- C3a acts with C5a to activate mast cells, recruit antibody, complement, phagocytes, and increase the tissue fluid in that area.
- C4a has the least anaphylactic activity.
Complement components and their functions:
| Complement Protein | Complement Fraction |
| Binding to Ag-Ab complex (Recognition unit) | C1q |
| Activating enzymes | C1r, C1s, C2b, Bb & D |
| Membrane binding proteins and Opsonin | C3b & C4b |
| Mediate inflammation | C5a, C3a, C4a |
| Membrane-attacking complex | C5b6789 |
Table XI – Complement proteins
Check Mechanism or Inhibitors of the Classical Pathway:
Nature has given a check mechanism, which controls unlimited damage caused by the activated complement system even to the innocent bystander cells. These inhibitors are:
- C1-inhibitor: It is a glycoprotein and also known as C1 esterase. It destroys C1r, C1s.
- The factor I: It is proteolytic enzymes, it cleaves C3b into iC3b (inactive form). It also destroys C4b.
- Vitronectin (S-protein): Prevents binding of C5, 6, 7 to the membrane.
- Decay Accelerating Factor (DAF): Prevents activation of C4.
- C4 binding protein: Prevents activation of C4.
- Factor H: It binds C3b and accelerates the destructive action of factor I.
ALTERNATIVE PATHWAY
- This is a primitive defense system. In this pathway, there is a bypass of C1, 4, 2 and there is direct stimulation of C3.
- This is predominantly a non-antibody initiated pathway.
- The activator of the alternative complement pathway are:
- Polysaccharides complex from the surface yeast cells.
- Bacterial polysaccharides and endotoxin.
- Aggregated immunoglobulins IgG2, IgA, and IgE.
- Zymosan.
- Inulin.
- The nonspecific activation is a major physiologic advantage because the host protection can be generated before the induction of a humoral immune response.
- C3a is considered the counterpart of the C2a of the classical pathway.
- C2 of the classical pathway resembles factor B of the alternative pathway.
- There is no role of C1, C2, and C4, but the alternative pathways have different protein which stimulates directly C3.
- Factor C3b and factor B combine to form C3b, B, which is then converted into C3 convertase (C3bBb).
- Factor Ba is a glycine richα-2-globulin believed to be physiologically inactive through the action of factor D.
- C3b, Bb complex can convert more C3 to C3b, in turn, it binds more factor B.
After the stimulation of C3 then there is a common pathway with the help of factor B and factor D. In the end, there is the formation of MAC (membrane attacking complex).
Alternative complement pathway and their components:
| Complement | Functions |
| C3b |
Binds to pathogen surface. Binds factor B for cleavage by D. |
| C3bBb | It is a C3 convertase. |
| C3b2Bb | It is a C5 convertase. |
| Ba | It is a small fragment and function is unknown |
| Bb | It is an active enzyme and C3 convertase. |
| C3bBb | It is a C3 convertase. |
| C3b2Bb | It is C5 convertase |
| C3bBb | It is a C5 convertase. |
| Factor D | Plasma serine protease cleaves factor B when it is bound To C3b to Ba and Bb. |
Table XII – Proteins of the alternative pathway
Positive Regulators for Alternative Pathways are certain substances, which enhance and give positive regulation of the alternative pathways. These are:
- Factor P (Properdin).
- Cobra Venom Factor (CoVF):- This complexes with Bb (CoVFBb) which acts as C3/C5 convertase. It is resistant to factor H & I.
Inhibitors of Alternative Pathways
These are natural check system or inhibitors.
- Factor H which binds to C3b.
- Factor H prevents the association of C3b and factor B.
- Factor H block the formation of C3b, Bb complex.
- Factor H competes with factor B for its combining sites on C3b and ultimately leads to the inactivation of C3 convertase.
- factor B and H occupy a common site on C3b.
- Polysaccharides are called surface activators and favor the uptake of factor B on the chain of C3b, and it will displace the factor H.
- The other controlling point is the amplification of C3b, Bb depends upon the stability of C3b-Bb convertase. C3b -Bb decay due to loss of Bb which has a half-life of roughly 5 minutes.
- If factor P combines with C3b-Bb and form C3b-Bb-P, then the half-life is increased to 30 minutes.
- The factor I which inactivate and cleaves C3b.
Complement Activation is an Amplification Process.
- Roughly 1200 C3b opsonin can deposit with a single molecule of IgM.
- A single IgM molecule cleaves one C1q and C1s and these will cleave 100 C4 molecules.
- 20 or 50 C4b cleaves about 100 C2 molecules.
- Many C5 molecules initiate MAC (membrane-attacking complex).
Complement Receptors
The most important function of complement is to facilitate the uptake and destruction of the pathogen by phagocytic cells. This occurs by specific recognition of bound complement components by complement receptors (CR) present on phagocytic cells.
Similar complement receptors are present on RBC and RBC play an important role in the clearance of soluble Ag + Ab complexes from the circulation by carrying these (Ag + Ab) complexes to a reticuloendothelial system like the spleen.
| Receptors | Present on |
| C3a and C5a Receptors |
|
| C1q |
|
| C3b |
|
| C4b |
|
Table XIII – Receptors present on various cells
Complement receptors and their functions:
| Receptor | Binds to | Present on | Function |
| CR1 | C3bC4b |
|
|
| CR2 |
C3d, iC3b, EBV |
B-cells |
|
| CR3 | iC3b | Macrophages, Monocytes, Neutrophils | Stimulate phagocytes |
| CR4 | iC3b | Macrophages, Monocytes, Neutrophils | Stimulate phagocytes |
| C1q | C1q | B-cells, Macrophages, Monocytes, platelets, and endothelial cells | Binds immune complexes to Monocytes |
Table XIV – Various receptors
Biological Functions of Complements:
| C1 and C4 | Virus neutralization. |
| C3b | Immune adherence, phagocytosis & take part in Arthus reaction. |
| C3a, C5a | Anaphylatoxin |
| C3a, C5a, and C6 C7 | Chemotaxis |
| C5 to C9 | Lysis |
| C8 and C9 | Cytotoxic action |
Human Diseases Associated with Complement Deficiency:
Deficiency of any component of complement leads to various diseases e.g.
| Complement | Diseases associated with complement deficiency |
| C1q deficiency | SLE, Nephritis & hypogammaglobulinemia |
| C1r deficiency | Renal diseases, SLE, recurrent infection, and Rheumatoid arthritis |
| C1s deficiency | SLE |
| C4 deficiency | SLE |
| C3 deficiency | Recurrent infections (pyogenic) |
| C5 deficiency | Recurrent infections + Gonococcal infections and SLE |
| C6 deficiency | Recurrent infections + Meningococcal infection |
| C7 deficiency | Recurrent infections + Glomerulonephritis |
| C8 deficiency | Recurrent infections + Gonococcal & Meningococcal infections |
Raised level of complement:
- Inflammatory process.
- Trauma.
- Myocardial infarction.
- In acute illnesses where complement raised as acute-phase proteins.
Decreased level of complement (deficiency of complements):
- Autoimmune diseases like:
- SLE.
- Rheumatoid arthritis.
- Systemic vasculitis.
- Essential mixed cryoglobulinemia.
- Infectious diseases:
- Arterioventricular shunts with infection.
- Subacute bacterial endocarditis.
- Gram-negative sepsis.
- Pneumococcal sepsis.
- Viral hepatitis B leading to viremia.
- Viremia in measles.
- Malarial parasite infestation.
- Deficiency of controlling proteins:
- These are quite uncommon primary immune deficiency <2%.
- The factor H deficiency.
- The factor I deficiency.
- Hereditary angioedema (C1 inhibitor deficiency).
Deficiency of complements and the diseases caused by:
Deficiency of complement Diseases caused by the complement deficiency C1s - SLE
- Angioedema
C1r - Glomerulonephritis
- Chronic infection
- SLE
- Dermatomyositis
- Vasculitis
- Necrotizing skin lesion
- Arthritis
C1q - SLE
- Decreased secondary to agammaglobulinemia
C2 - Recurrent pyogenic infection
- SLE
- Membranoproliferative glomerulonephritis
- Discoid lupus erythematosus
- Dermatitis herpetiformis
- Dermatomyositis
- Synovitis
- Purpura
- Hodgkin’s disease
- Chronic lymphocytic leukemia
- Polymyositis
C3 - Recurrent pyogenic infection
- SLE
- Arthritis
- Skin rash
C3b inactivator - Recurrent pyogenic infection
- Urticaria
C4 - SLE
- Vasculitis
- Dermatomyositis like syndrome
C5 - Recurrent infection (Neisserai)
- SLE
C6 - Gonorrheal infection
- Meningococcal infection
- SLE
- Scleroderma
- Vasculitis
- Raynaud’s phenomenon
C7 - Raynaud’s disease
- Renal disease
- Neisseria infection
- SLE
- Vasculitis
- Scleroderma
C8 - Glomerulonephritis
- SLE
- Neisseria infection
- Xeroderma pigmentosa
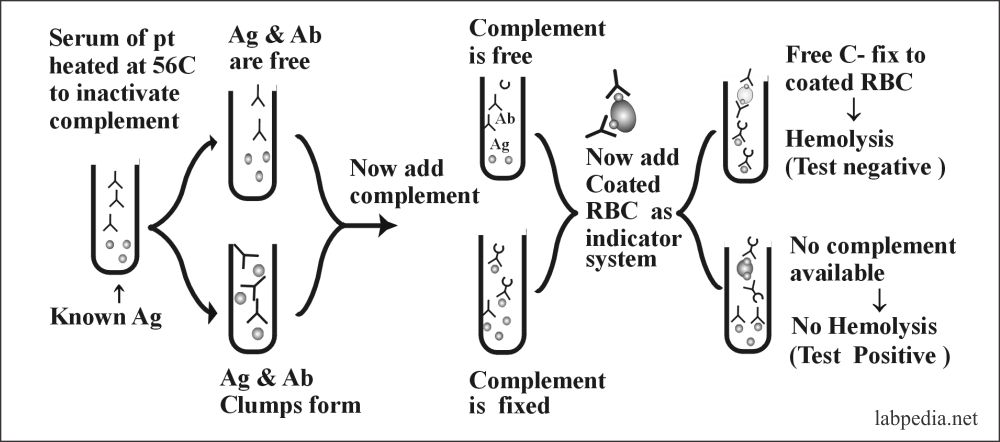
Complement Fixation Test
The complement may be used as a diagnostic tool. The basic principle is its activity which leads to hemolysis of RBC. In this test, we need:
- Serum of the patients.
- Complement
- Indicator system which consists of antibody-coated RBC.
- Known antigen.
This test is used to find Unknown Antibody or Antigen. The following diagram shows the basic mechanism.
This test may be manipulated by making dilution of serum or complement or one can have known antibody and unknown antigen or vice versa. Even the titer of antibody or concentration of complement may be estimated by serial dilution.
Use of Complement fixation test
- This test can be used to diagnose various diseases e.g Pneumococcal pneumonia, syphilis and etc.
- With the help of this test, one can quantitate antigen or antibody.
- This test was very famous for the diagnosis of syphilis.