Chapter 4: Antibody (Ig), Immunoglobulins Structure
ANTIBODY (Ig)
Definition
- Antibodies are glycoproteins called immunoglobulins.
- The term immunoglobulin has replaced the γ-globulins because not all the antibodies have gamma electrophoretic mobility.
- Antibodies are found in the plasma and other body fluids like tears, saliva, milk, and colostrum.
Porter and Edelman described in detail about antibodies in 1950 and they were awarded Nobel Prize.
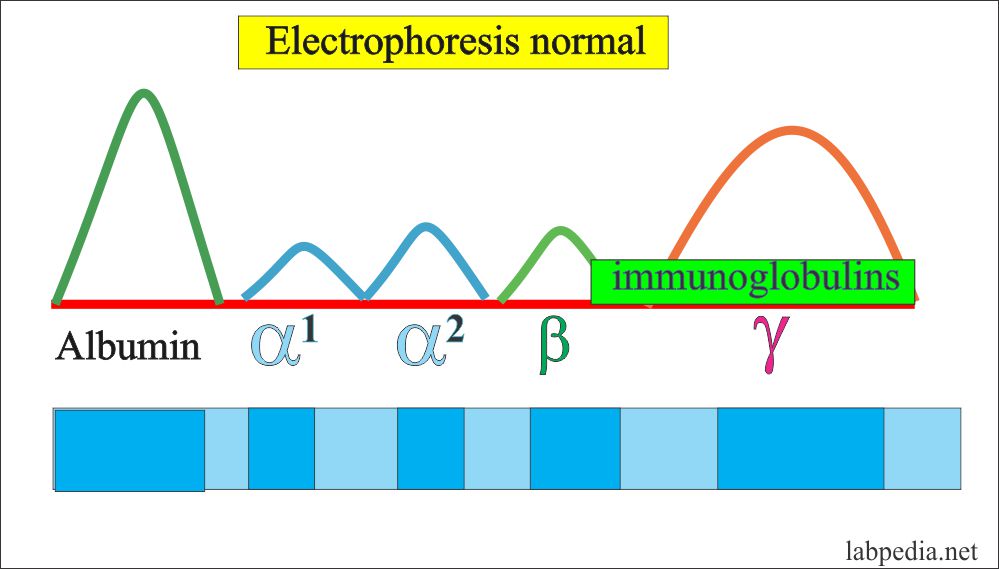
The antibody band is mainly in the γ-fraction and small amount in β-Portion. So these are mainly globulin. γ- globulin can be synthesized by the liver, while antibodies are produced by immune cells so these are called immunoglobulin (Ig) as related to the immune system and produced by activated B-lymphocytes and plasma cells.
Immunoglobulin (Ig) consists of :
- Immunoglobulins are glycoproteins and consists of:
- Polypeptide = 82 to 96%
- Carbohydrate = 4 to 18%
- Immunoglobulins (Ig) are 20% of the total plasma protein.
- Immunoglobulins (Ig) is a bifunctional molecule:-
- They bind with antigen.
- They initiate a secondary immune response.
Evolution of Immunoglobulins (Ig) Molecule
- Ig molecule consists of a heavy chain (H) and light chains (L).
- Heavy chain (H) gene present on Chromosome 14.
- The light chain (L) lambda (l) gene is present on chromosome 2.
- The light chain (L) kappa (k) gene is present on chromosome 22.
- Ig molecule consists of 12 basic units’ structures or units.
Therefore, Ig consists of 4 polypeptide chains, one pair is a light chain and 2nd pair is a heavy chain.
Heavy chain (H) :
- Molecular weight is 53000-75000.
- Consists of 446 aminoacids.
- 1-121 aminoacids are variable.
Light chain (L):
- The molecular weight is 22500.
- Consists of 214 amino acids.
- 1 to 107 amino acids are variable.
Immunoglobulins (Ig) have 5 different heavy chains and these are given Greek letters.
- Gamma = Heavy chain (γ)
- Alpha = Heavy chain (α)
- Mu = Heave chain (μ)
- Delta = Heavy chain (δ)
- Epsilon = heavy chain (ε )
Based on heavy chains, these Ig are classified as follows:
γ = Heavy chain = IgG
μ = Heavy chain = IgM
ά = Heavy chain = IgA
δ = Heavy chain = IgD
ε = Heavy chain = IgE
Light chains are of the following two types, with the ratio of 2:1.
- Kappa (κ)
- Lambda (λ)
Always the Ig molecule will have one type of light chain on both arms either kappa or lambda. Never both (Κ or λ) are on the same Ig molecule.
These immunoglobulin classes differ from each other on the basis of molecular weight, sedimentation coefficient, and carbohydrate contents.
Division of Ig Based on Molecular Weight
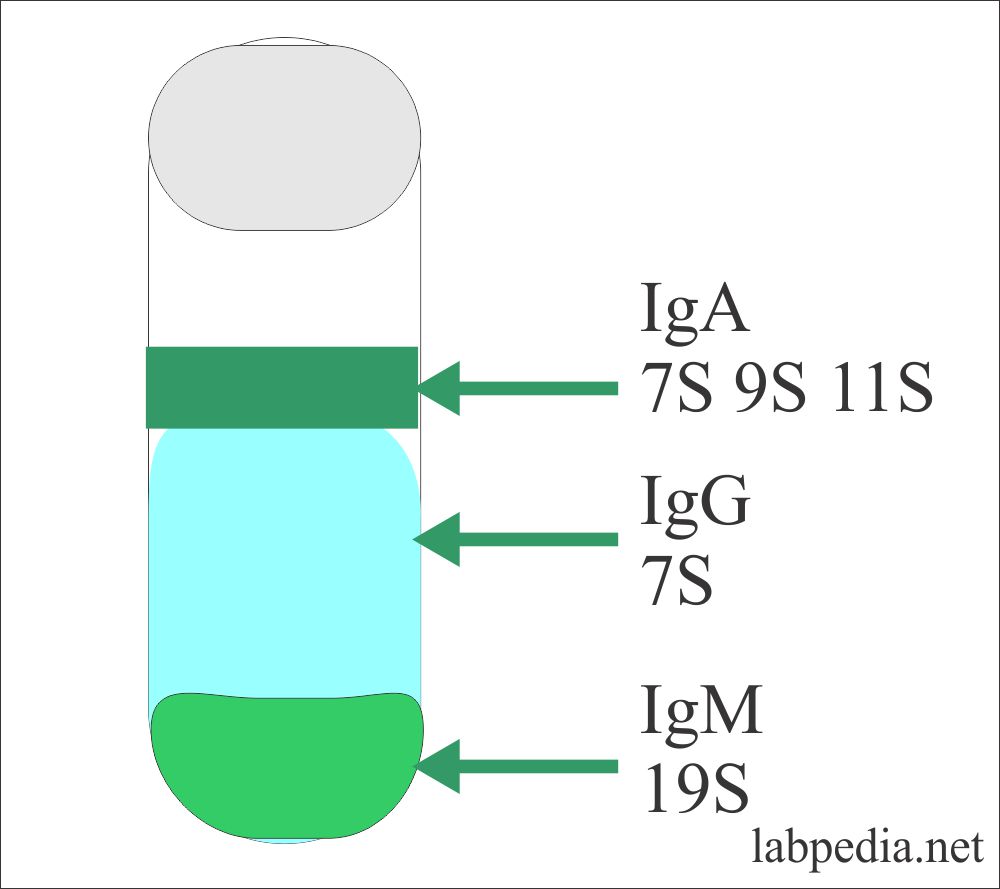
This procedure was adopted by Svedberg where he gave ultracentrifugation for a definite period of time and Ig was separated into different layers according to their molecular weight called Svedberg unit = S. The heaviest IgM was at the bottom and labeled as 19S (Svedberg unit = S). The major portion was by IgG, which is 7S, and IgA is a mixture of 7S, 9S, and 11S.
STRUCTURE
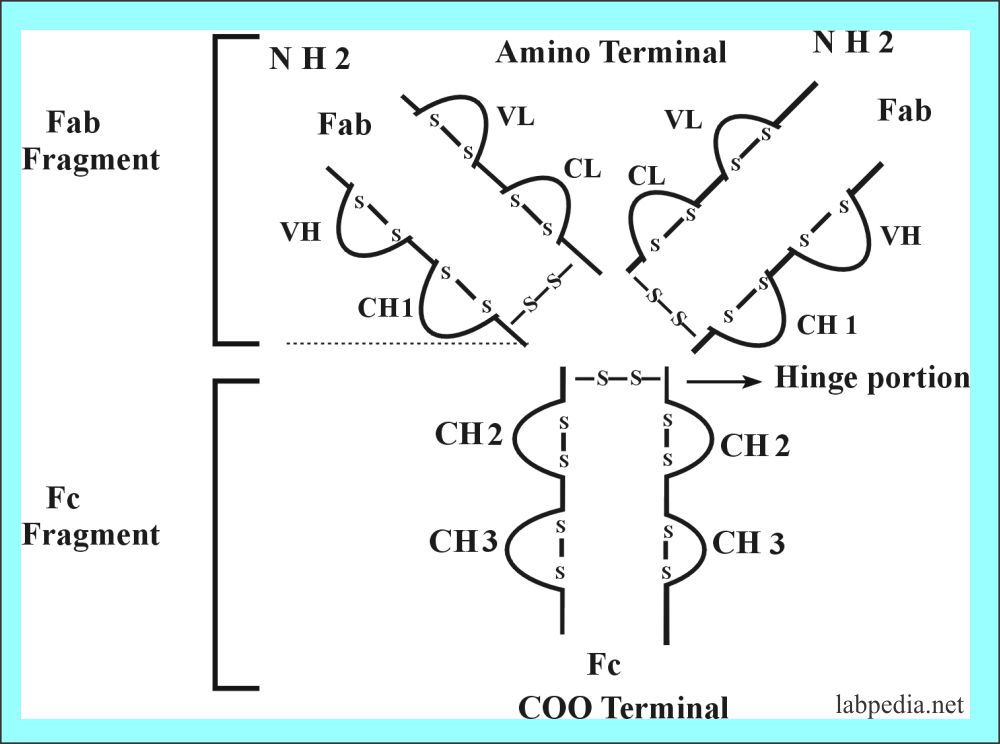
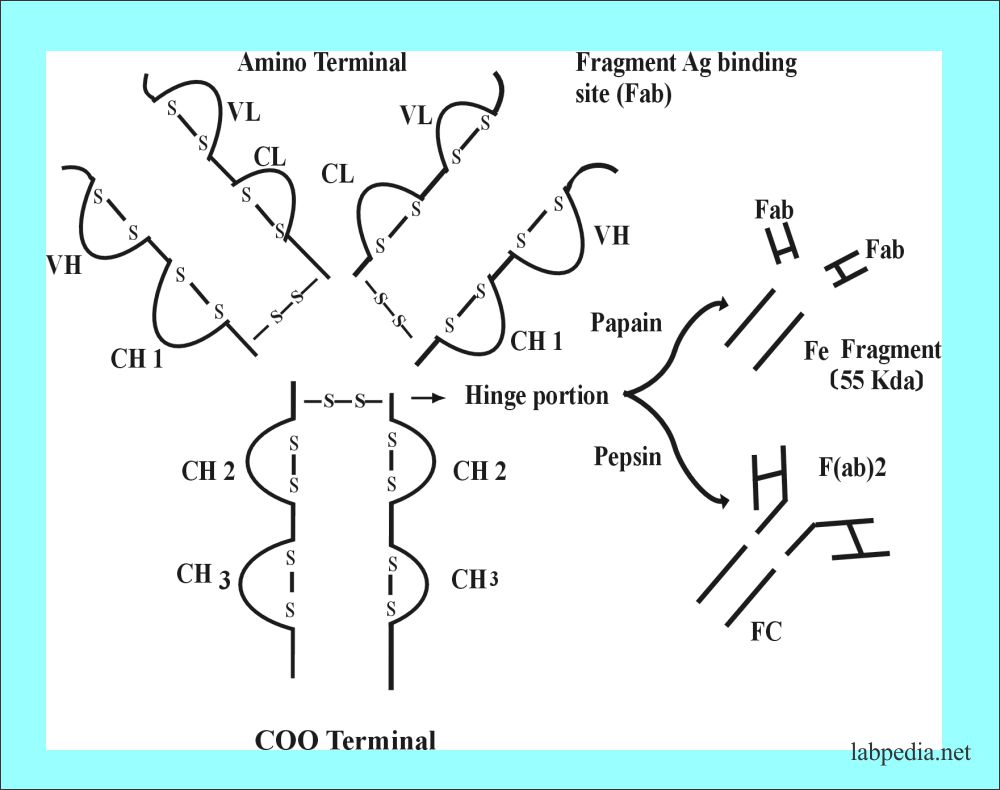
The basic unit of Ig consists of 4-polypeptide chains linked by a disulfide bond; one pair is light and one pair is heavy.
The disulfide bond is -S-S- and these hold together 4 polypeptide chains. These are:
Interchain :
- H-H
- H-L
- L -L
Intrachain:
- L chain has 2 bonds.
- γ, α, and δ has 4 bonds.
- μ and ε have five bonds
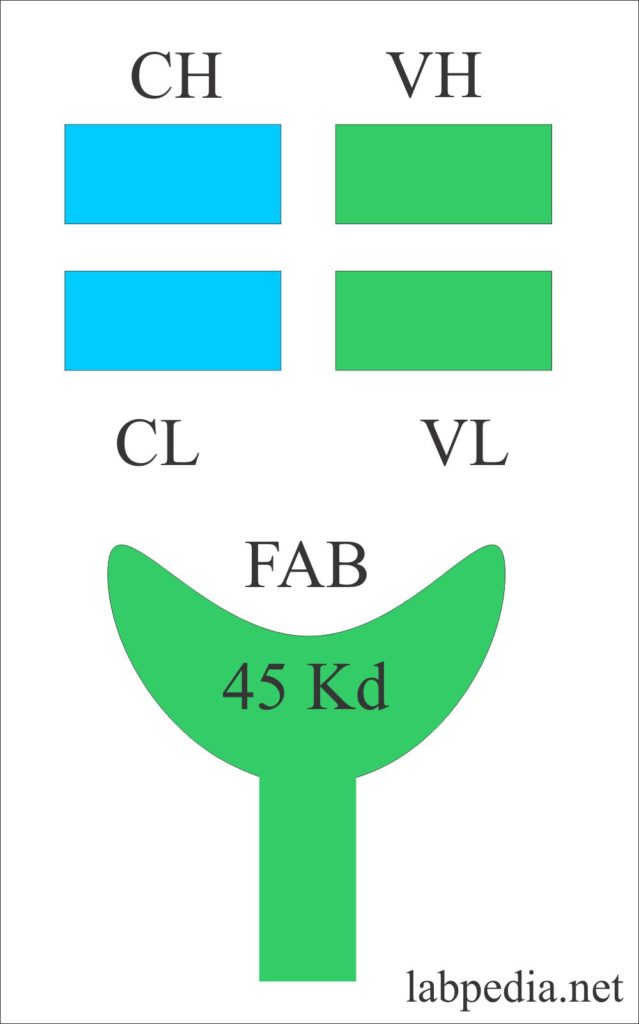
Fragment Antigen Binding Site (Fab)
Fragment antigen-binding site or Fab with a molecular weight of 45 kDa. It consists of a variable portion of the light chain and heavy chain with a constant portion of the light chain and heavy chain.
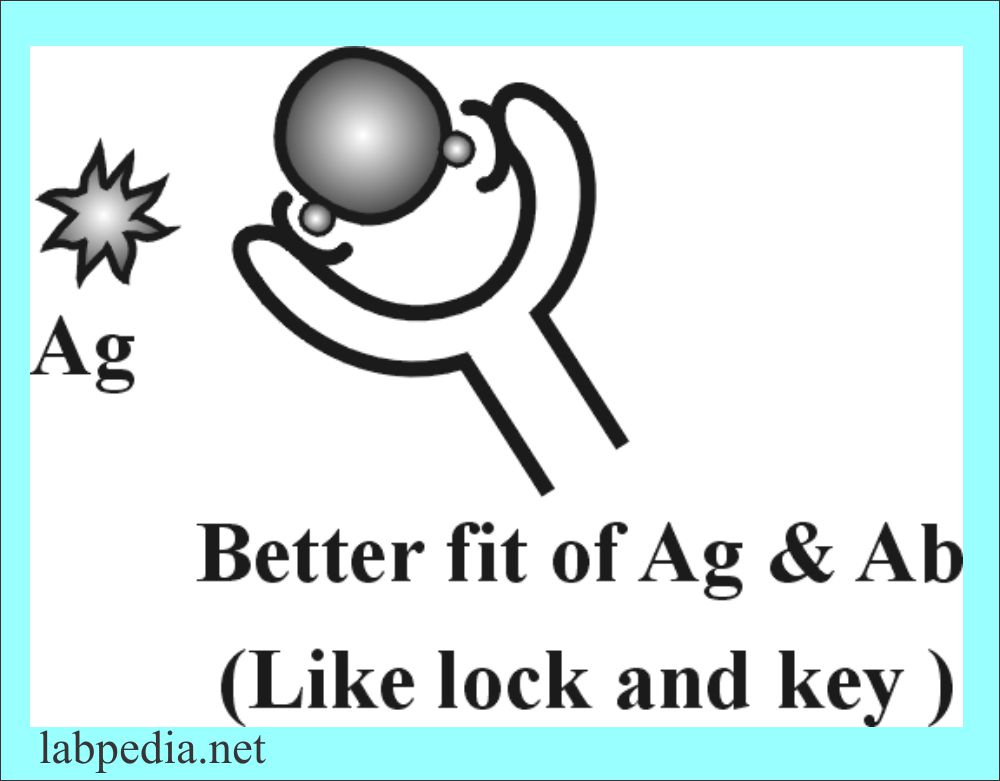
Because of variability in the position of amino acid in the variable portion, it can change shape according to the shape of the antigen, because antigen, antibody interaction is shape dependants. It is demonstrated in the following diagram that Ab better fit will give a better Ag-Ab reaction.
Fragment Crystalisable (Fc)
Fc portion can be crystallized into pure protein crystals. Fc portion gives some characteristic features to these immunoglobulins like:
- Porter showed that an antigenic determinant that distinguishes one Ig from the other is located on the Fc portion, so it gives Biological identity.
- IgM and IgG can activate complement.
- IgA may be secreted into body fluids and mucous membranes.
- IgE fixes to specific receptors on mast cells and basophils.
- IgG can cross the placental barrier.
- IgD acts as a membrane receptor.
- Fc portion is responsible for opsonization and phagocytosis.
When the Ig molecule is treated with papain or pepsin, then it is cleaved at different sites.
Variable Portion
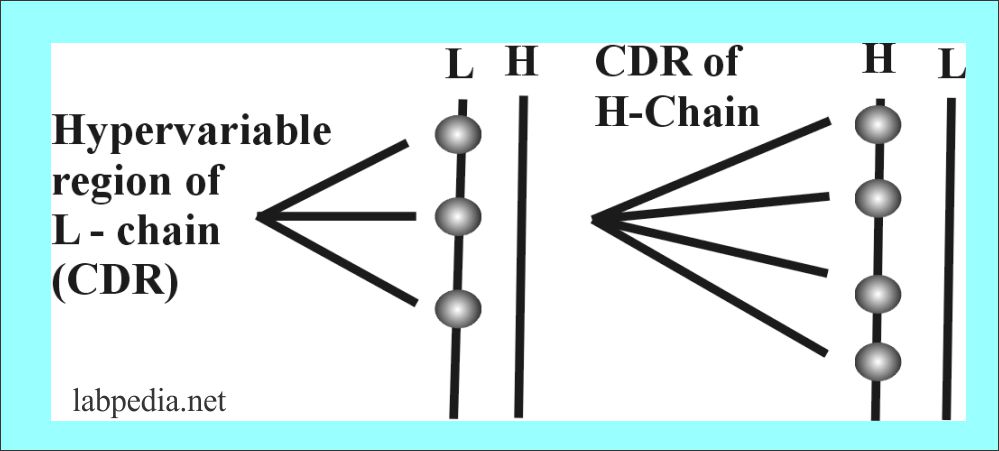
There are hypervariable areas in the variable portion of Ig which consists of 10 aminoacid residue and these small portions are called complementarity determining region (CDR), also called a Hot spot.
| CDR (Complimentarity regions) | Heavy chain | Light chain |
| 1 | 30 to 35 Amino acid | 24 to 34 Amino acid |
| 2 | 50 to 65 Amino acid | 50 to 56 Amino acid |
| 3 | 96 to 102 Amino acid | 89 to 97 Amino acid |
The variability is critical for generating the potential to bind to more than 107 different antigenic structures.
Domains
In Ig, domains are the area named according to the chain, (H or L). They are numbered as below:
| Domain | Light chain | Heavy chain |
| Variable domain | VL | VH |
| Constant domain | CL | CH1. CH2, CH3 |
Hinge Portion
This is the middle of the Ig molecule, which allows some freedom to two arms bearing antigen-binding sites (Fab). This gives the flexibility of Ig to bind two antigenic sites at the same time.
Paratope
This is the portion of antibody in the Fab portion which combines with the antigenic determinant (Epitope). There is a folding of polypeptide chains bringing the CDR portion of heavy and light chain in close proximity to Epitope and creates a three-dimensional structure that fits the epitope. Epitope and paratope are just like lock and key.
IMMUNOGLOBULIN ANTIGENCITY
There are antigenic areas on the Ig molecule, because of these areas Ig can initiate Ab formation and become antigenic. Differences and similarities between Ig molecules are reflected in there antigenic properties, these are as follows:
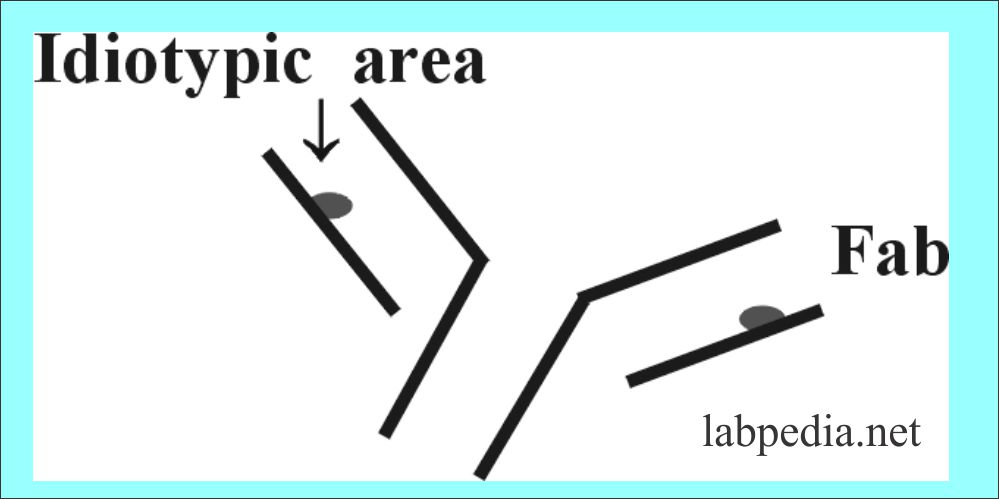
Idiotype
This is the antigenic area in the Fab portion of an immunoglobulin. When Ig are injected into another animal. There will be Ab formation against the Ig (Ab) and that will be against the idiotypic area. These are antigenic determinant present in hypervariable regions or CDR.So there will be Antibody formation against Antibody (anti-anti Ab)
Isotype
These are present on all molecules of a particular immunoglobulin class or heavy or light chain type in a species and are called Isotype. e.g. ε-heavy chain has antigen peculiar to the IgE molecule which is present in all of the IgE molecule in members of that species.
Allotype
The Immunoglobulins have genetically determined differences between individuals, and are called Allotype. These allotypes are denoted by the chain affected e.g. Gm, Em. These are polymorphic forms of H and L chains and localized in the constant portion.
FUNCTIONS OF ANTIBODY
The Antibody has a range of functions and uses both in biological defense and as a clinical tool.
- Host Defence
- Defend against microorganisms (bacteria, viruses).
- The first antibody, which appears, is IgM and followed by IgG.
- In fetal life, IgM appears at 6 months of intrauterine life.
- Mother (milk) is a rich source of IgA and IgG.
- Recruits another component like complement which destroys the antigen.
- Cause neutralization of toxins.
- Leads to the removal of foreign antigen from the circulation.
- Clinical Medicines
- Used in the diagnosis of diseases and monitoring of the progress of diseases.
- It can be used as passive therapy by giving pooled antibodies. It also can be used for prevention.
- Diagnosis and Research
- In the laboratory sciences, these can be used for diagnostic and research purposes.