Chapter 16: Autoimmunity, Immunologic Tolerance, and Mechanism of Autoimmune Diseases
AUTOIMMUNITY
HISTORICAL BACKGROUND
Ehrlich Reported self-destructive process occurs directed by one’s immune system and called it “Horror autotoxicus.”
Autoimmunity is evidence of an immune reaction to self components (self-antigen) in the absence of overt disease.
The term autoimmune response refers to demonstrating the auto-antibodies directed to a self-antigen or reactivity of the lymphocytes sensitized to the “self” antigen. At the same time, the autoimmune disease results from tissue damage by the autoimmune response.
All of us can mount autoimmune responses, and in some circumstances, this may be a healthy, physiological reaction. This spectrum of the immune response from physiological may reach the pathological range.
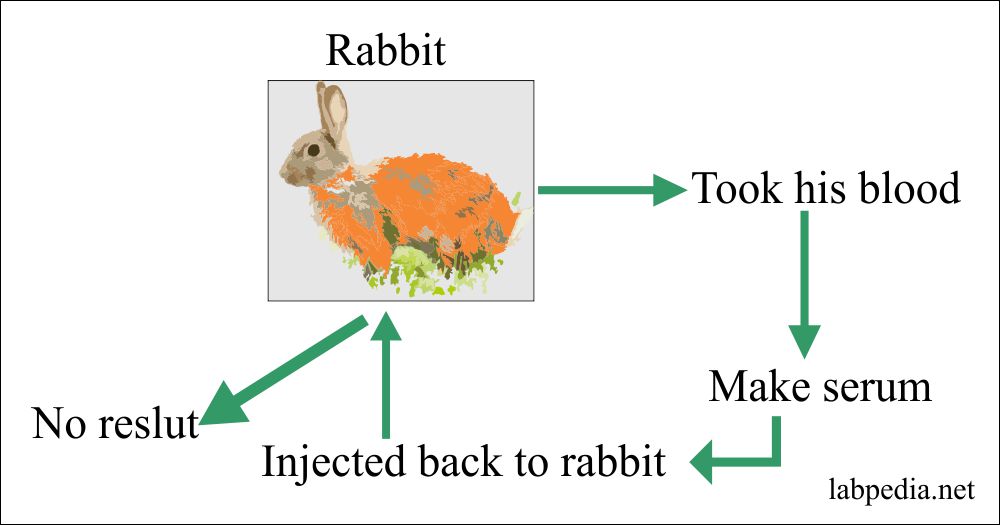
1898 Bordet experimented on Rabbit. He transfused RBC to Rabbit and then took his serum and injected it into Rabbit, but he could not get some positive response.
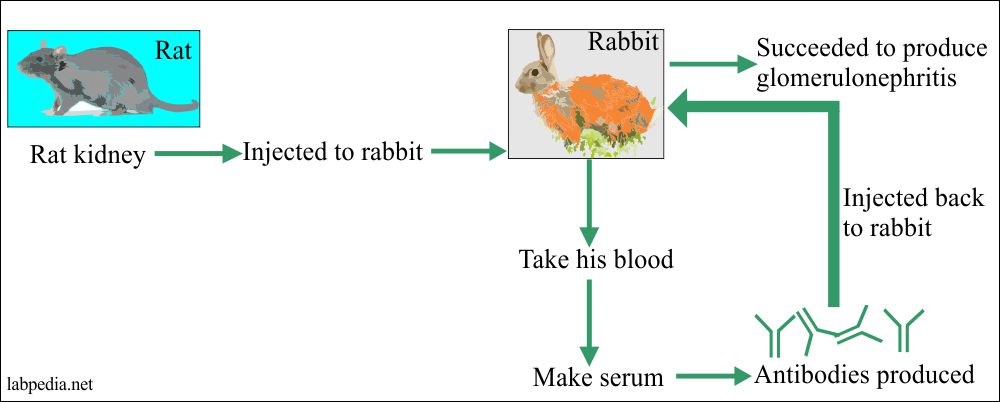
1929 Masugi succeeded in producing glomerulonephritis by injecting the Rat kidney to Rabbit, and Abs was injected back to the same Rabbit.
1900 Paul Ehrlick gave the idea of harmful immunity “Horror autotoxicus.” His son, two years old, died due to diphtheria antitoxin serum injection after a few minutes.
Von Pirquet. First time introduce the concept about the collision of antigen and antibody.
Dixon. He explained that regardless of Antigen’s source, if this results in tissue damage in the host is called autoimmune disorders.
Graber gave the concept that auto-Abs are of two types:
- 1st group has a biological function as taxis for transportation and disposable of Antigen.
- 2nd group Abs cause damage to the host.
It is believed that 5% of us will develop autoimmune diseases during our life.
Definition of autoimmunity and autoimmune diseases:
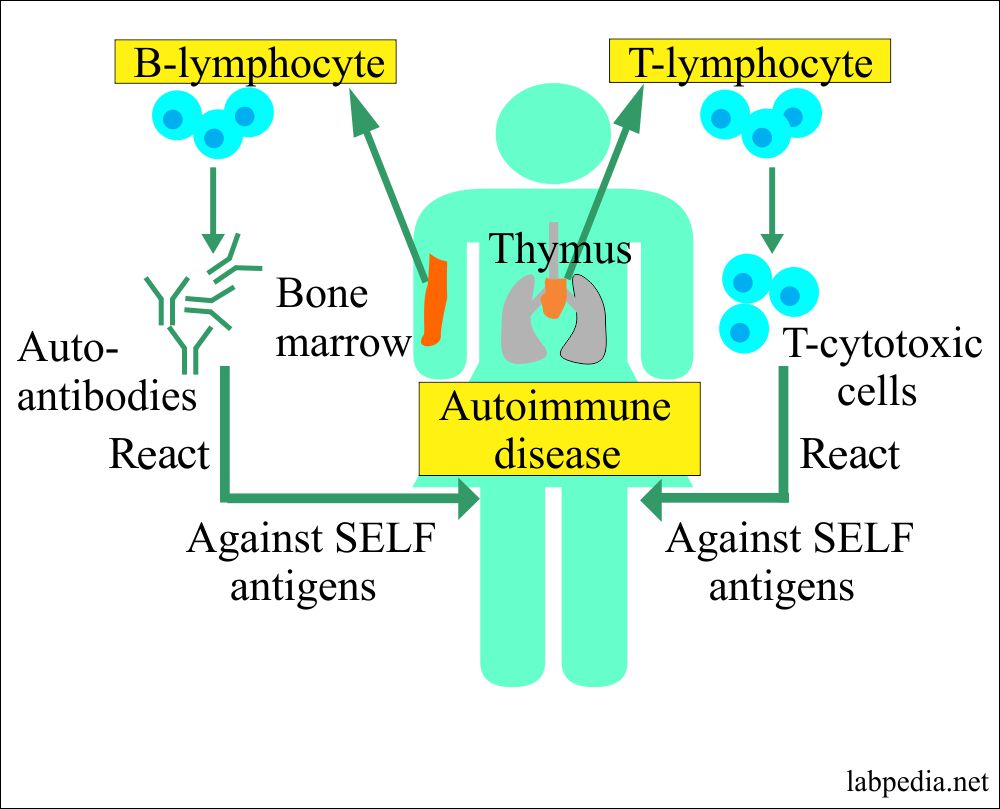
Autoimmunity is the breakdown of the immune system’s ability to differentiate between self and nonself.
Autoimmune disease is used when demonstrable immunoglobulins (autoantibodies, B-lymphocytes activation) or cytotoxic T-lymphocytes display specificity self-antigens autoantigens and give rise to the pathogenesis of the disease.
The spectrum of Autoimmune Disease
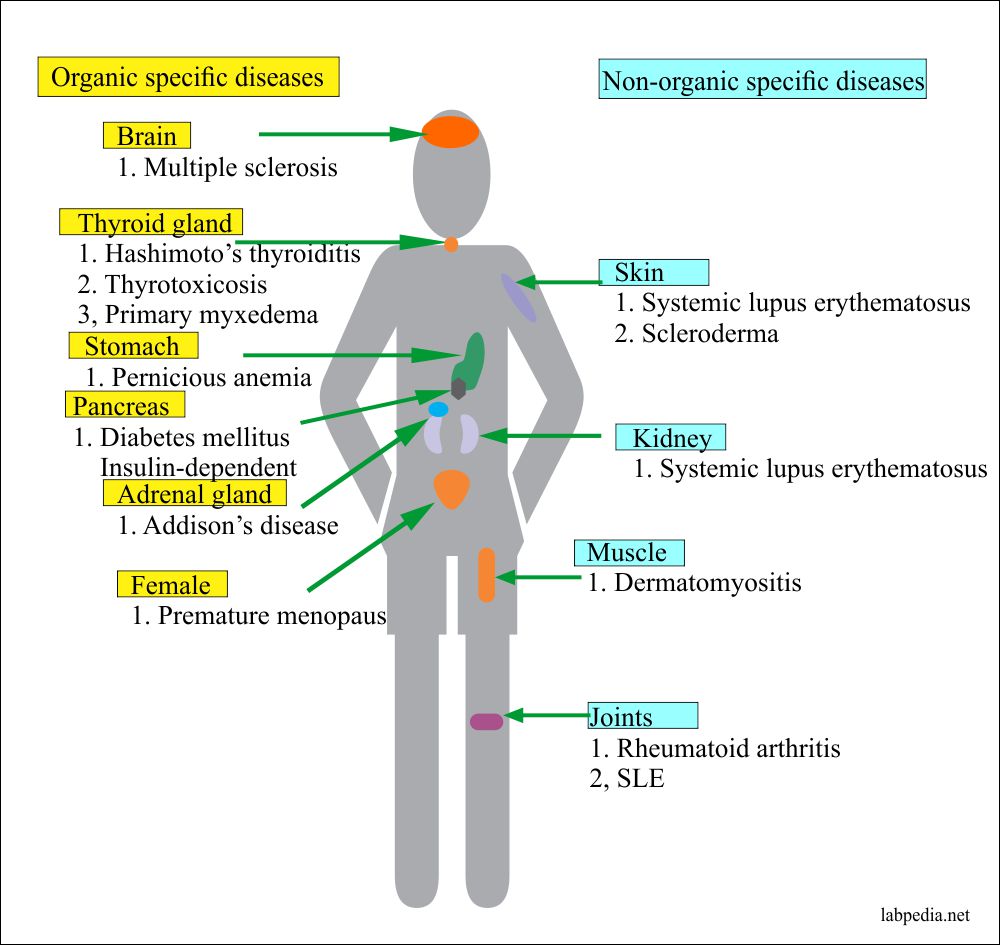
There are organ-specific autoimmune diseases where Antibodies are described against one or more specific cytoplasmic constituents, plasma membrane structure, or secreted products of the cells.
While non-organ specific auto-immunity is directed against structures common to many tissues and found throughout the body, e.g., nuclear components, mitochondrial proteins, or constituents of the muscles, there may be overlaps of disease in the same group in some individuals, e.g., patients with Hashimoto’s thyroiditis have a much higher incidence of Pernicious Anaemia.
|
||||||
| Hashimoto’s thyroiditis | Autoimmune hemolytic anemia | Good Pasteur syndrome | Primary biliary cirrhosis | Systemic lupus erythematosus | ||
| Primary myxedema | Idiopathic thrombocytopenic purpura | Myasthenia gravis | Chronic active hepatitis | Discoid lupus erythematosus | ||
| Thyrotoxicosis | Idiopathic leucopenia | Juvenile diabetes | Cryptogenic cirrhosis | Scleroderma | ||
| Pernicious anemia | – | Pemphigus Vulgaris | Ulcerative colitis | Rheumatoid arthritis | ||
| Addison’s disease | – | Sympathetic ophthalmia | Sjogren syndrome | – | ||
| Premature menopause | – | – | – | – | ||
| Male infertility | – | – | – | – | ||
Table XXIII – Overlap Between Organ-Specific and Non-Organ Specific diseases
Overlap of Auto-antibodies:
There is also an overlap of auto-antibodies in the same group; e.g., in patients with thyroid diseases, 30% may show at the same time antibodies against parietal cells while thyroid antibodies are found in 50% of pernicious anemia cases.
Similarly, Rheumatoid arthritis patients are clinically associated with a clinical picture of SLE.
Autoimmune diseases and their possible source of antigens:
| Disease | Antigen |
| 1. Hashimoto’s thyroiditis |
|
| 2. Primary Myxedema |
|
| 3. Thyrotoxicosis |
|
| 4. Pernicious anemia |
|
| 5. Addison’s disease |
|
| 6. Premature menopause |
|
| 7. Juvenile diabetes |
|
| 8. Good Pasteur Syndrome |
|
| 9. Pemphigus Vulgaris |
|
| 10. Myasthenia gravis |
|
| 11. Autoimmune Hemolytic Anaemia |
|
| 12. Idiopathic thrombocytopenic purpura (ITP) |
|
| 13. Primary biliary cirrhosis |
|
| 14. Chronic active hepatitis |
|
| 15. Ulcerative colitis |
|
| 16. Sjogren syndrome |
|
| 17. Rheumatoid arthritis |
|
| 18. Scleroderma |
|
| 19. Dermatomyositis |
|
| 20. Systemic lupus erythematosus |
|
Table XXIV – Autoimmune Diseases with their Possible Source of Antigen
The autoimmune disease could be considered one end of a spectrum of autoimmunity stretching from physiology to pathology. However, the fact that the vast majority suggests that regulatory mechanisms are critical. It is a state of balanced, physiological autoimmunity that may be described as Tolerance.
Somebody may define autoimmune diseases as a pathological process leading to the breakdown of Self-Tolerance.
Before discussing autoimmune diseases, we have to make a clear concept, “What is Immunologic Tolerance,” particularly “Self Immunologic Tolerance.”
IMMUNOLOGIC TOLERANCE
Definition:- Immunologic Tolerance is a state in which an individual is incapable of developing an immune response to a specific antigen.
Immunologic tolerance prevents harmful reactivity against the body’s own tissue.
Immunologic Tolerance may be:
- Present at birth–prenatal tolerance.
- It is acquired or present in adult age.
Immunologic Tolerance may be:
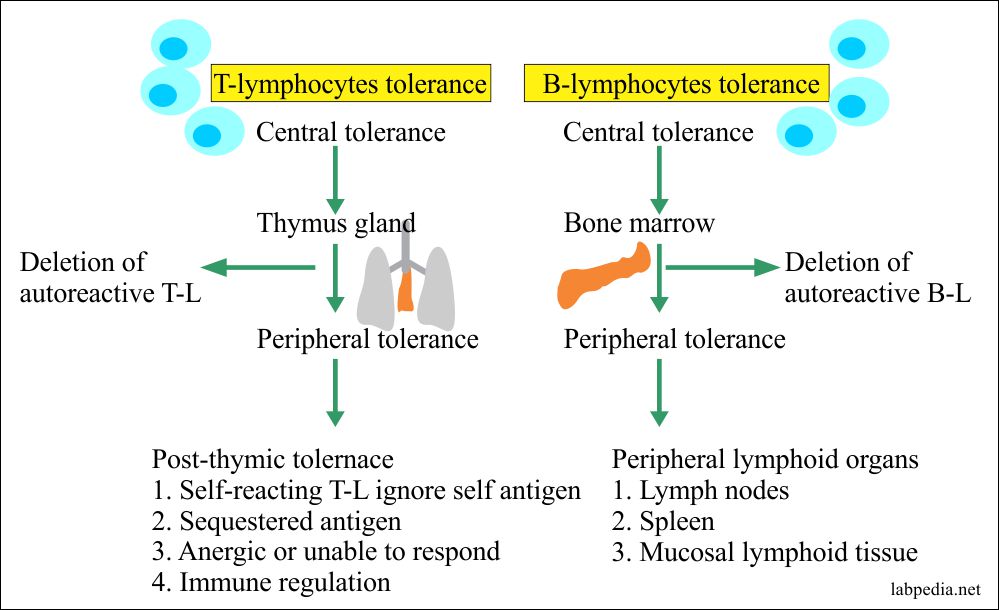
- Central–induced centrally in the primary lymphoid organs, e.g., thymus for T-L tolerance and bone marrow for B-L tolerance. This is the first stage of tolerance.
- Peripheral–Tolerance in mature B and T-cells. This is acquired in the Lymph nodes, spleen, and mucosal lymphoid tissues. This is the second stage of tolerance.
Owen:– 1945 – A veterinary surgeon explained that dizygotic twins sharing blood supply in-utro could tolerate their blood later on.
Medawar:– 1960 – He experimented on the above hypothesis and introduced antigen in-utro, and later on, this animal was tolerant of the same antigen when introduced in-utro. He got the Nobel Prize.
Self Immunologic Tolerance
The following are the different explanations and theories for the development of self Immunologic Tolerance.
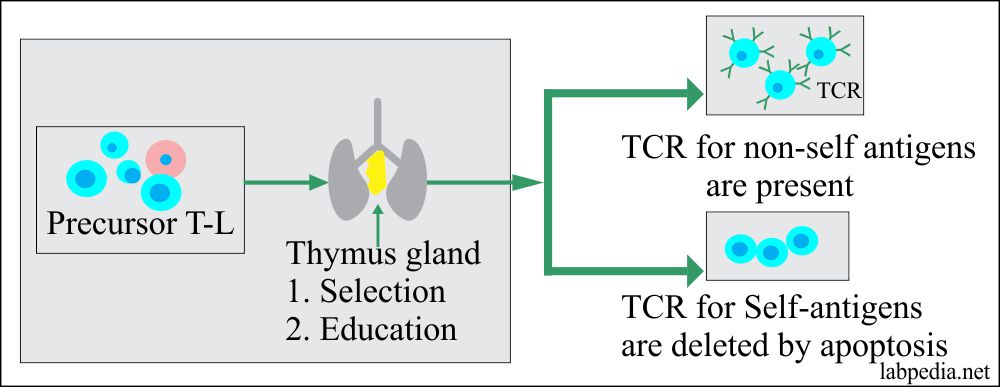
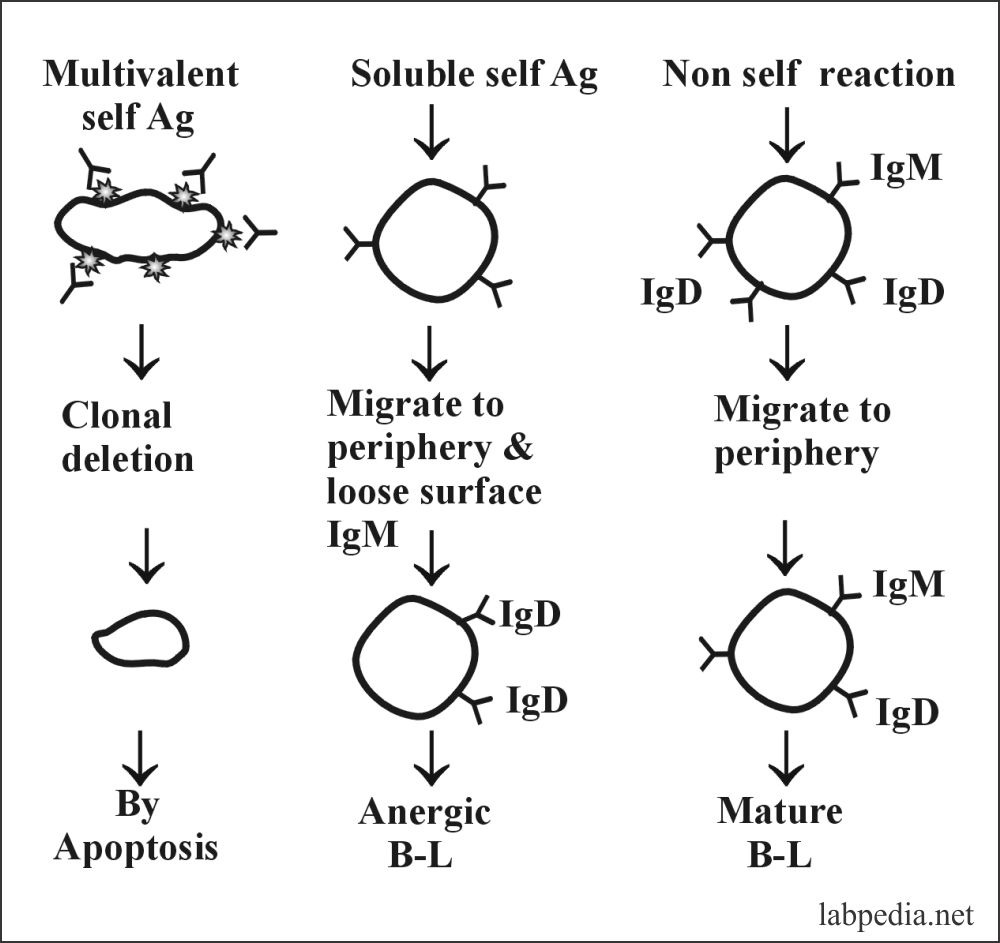
1. Burnet Clonal Deletion Theory: It is believed that self-antigen reacting B and T-L clones are deleted from the circulation, e.g., during the development stages of lymphoid tissue.
- This process takes place in the thymus gland.
The same process takes place for B –Lymphocytes. That self Antigen bearing B-cells are deleted by apoptosis in the bone marrow.
There was an objection to clonal deletion theory because, later on, self-antigen reacting clones of lymphocytes are present in our circulation. This theory was modified that there is clonal anergy.
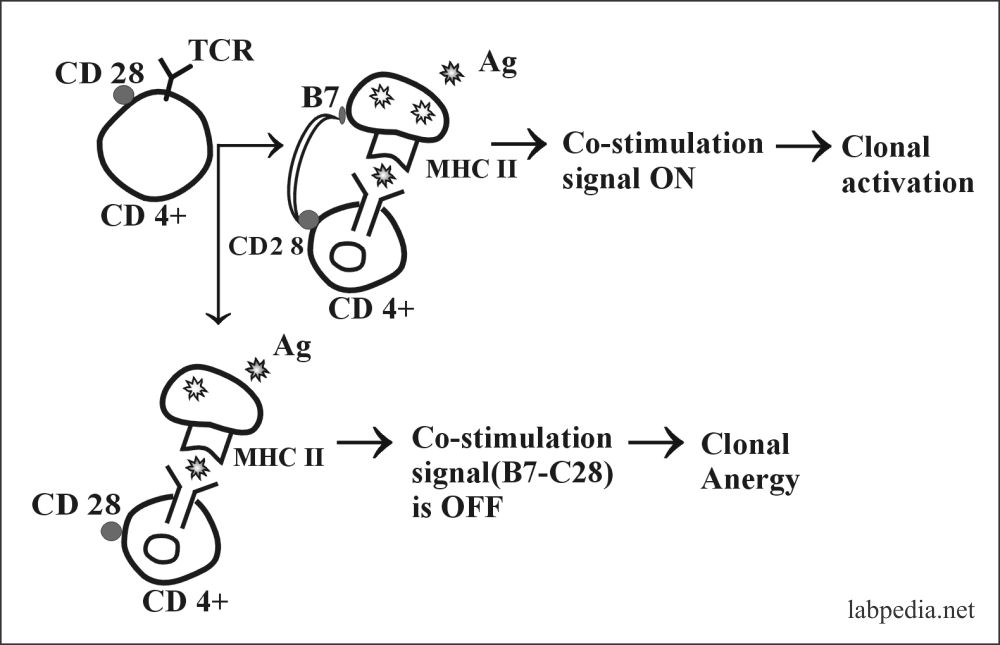
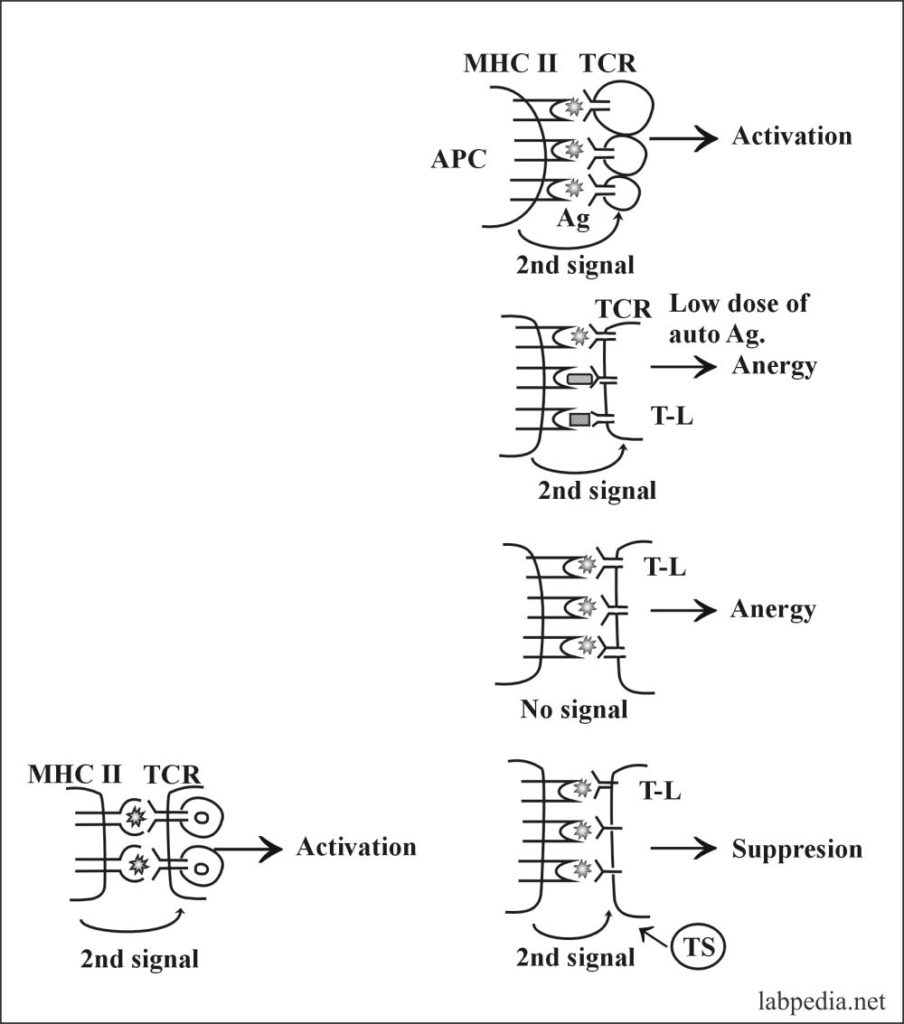
2. Clonal Anergy:- This is prolonged irreversible functional inactivation of lymphocytes. This is also called clonal purging or clonal silence, clonal abortion, or obligatory paralysis.
- Ag–Specific T – L (CD4+) requires two signals:
- Recognition of Ag-peptide with MHC-II.
- The second co-stimulating signal provided by APC–T – L associated molecule, CD28, must bind to its ligand B7 on APC.
3. B-L also has clonal anergy (B-lymphocyte Tolerance):-
B-lymphocytes during maturation, Ag-receptor (IgM) after self-Ag recognition are endocytosed, and Ag-receptor (IgM) is not expressed.
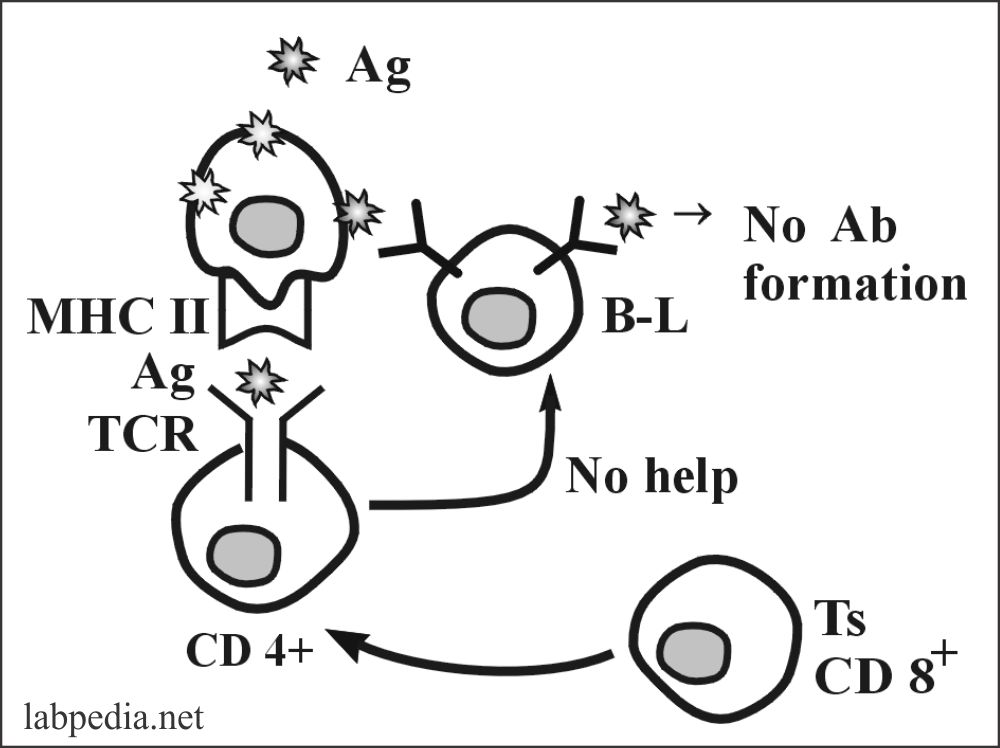
4. Peripheral Suppression by Ts (CD8+) Cells
Ts(CD8+) suppress the immune response by producing cytokines. These are:
TGFβ1 – It down-regulates many immune responses. Directly suppress:
- Th-cells.
- B-lymphocyte.
- Prevents autoimmune response.
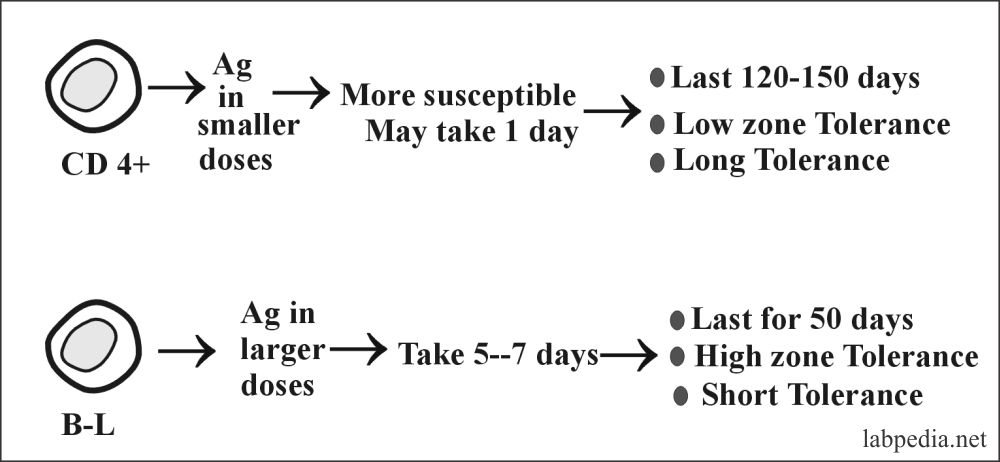
5. Tolerance at Adult Age (Acquired or Peripheral Tolerance):–
- B and T lymphocytes can be made tolerant by giving antigens in various doses.
- The (CD4+) cells are made tolerant by giving antigens in smaller doses, while B-L is made tolerant by giving antigens in larger doses.
- So peripheral T-L suppression may be due to:
- Low antigen concentration.
- APC, the presenting cell, is incapable of a second co-stimulatory signal.
- Regulatory Ts cell suppresses the autoreactive T-L.
AUTOIMMUNE DISEASES AND THEIR MECHANISM:
There are three hypotheses for autoimmune diseases are:
- They have forbidden clone theory.
- The Sequestered antigen theory.
- Immunologic deficiency.
This is a breakdown of self immunologic tolerance leading to autoimmune diseases. The different mechanisms through which T and B cell tolerance operate lead to an obvious conclusion: Several other pathological processes could break tolerance and lead to autoimmunity.
Tolerance to self-antigen may be broken by:
- Defect in immune regulatory pathways.
- The presence of Ag similarities between pathogenic organisms and self-proteins.
- The provision of new T-cell epitopes to bypass tolerant T-cells.
- The release of “hidden” self-antigen.
- The so-called “aberrant” expression of the MHC class II molecule.
- The influence of cytokines.
- Circumstantial evidence of familial tendency.
- Lymphocytic infiltrate.
- MHC–association.
- Clinical improvement with immunosuppressive therapy.
Criteria for Autoimmune Diseases:
- Presence of autoimmune disease.
- Clinical or experimental evidence of the disease is not due to tissue damage, e.g., when administering thyroglobulin will produce thyroiditis.
- Absence of any other evidence of disease.
MECHANISM OF AUTOIMMUNITY
It has been discussed in various books on immunology in different ways. This book discusses how it may be easy to reproduce and have a basic autoimmune disease mechanisms concept.
If given appropriate circumstances, the potential for autoimmunity is frequently present in all immunocompetent individuals because the lymphocytes that are potentially reactive to self-antigen still exist in the body.
1.The primary mechanism may be:
- Evasion of the standard tolerance to self-antigen.
- Hidden antigen (sequestered antigen).
- Alteration of antigen by chemical, drugs, or infections.
- Breakdown of tolerance mechanism.
- Agents are affecting antibody-forming cells, e.g., chemicals, drugs, infections.
- Genetically determined lake of efficiency of tolerance, e.g., TS activity.
- Stimulation of pre-existing B-L, e.g., by infection.
- Genetic factors.
- The presence of some HLA antigens is associated with an increased risk of autoimmune disease.
- There is a familial tendency for familial aggregates of the autoimmune disease.
- Age of the patients.
- The presence of autoantibodies increases with increasing age. The peak is around 60 to 70 years of age.
- Exogenous factors.
- Exogenous factors are leading to autoimmune diseases like:
- Ultraviolet rays.
- Viruses.
- Infections.
- Drugs.
- Mostly these factors alter the antigens, which are taken as nonself by the immune system.
- Exogenous factors are leading to autoimmune diseases like:
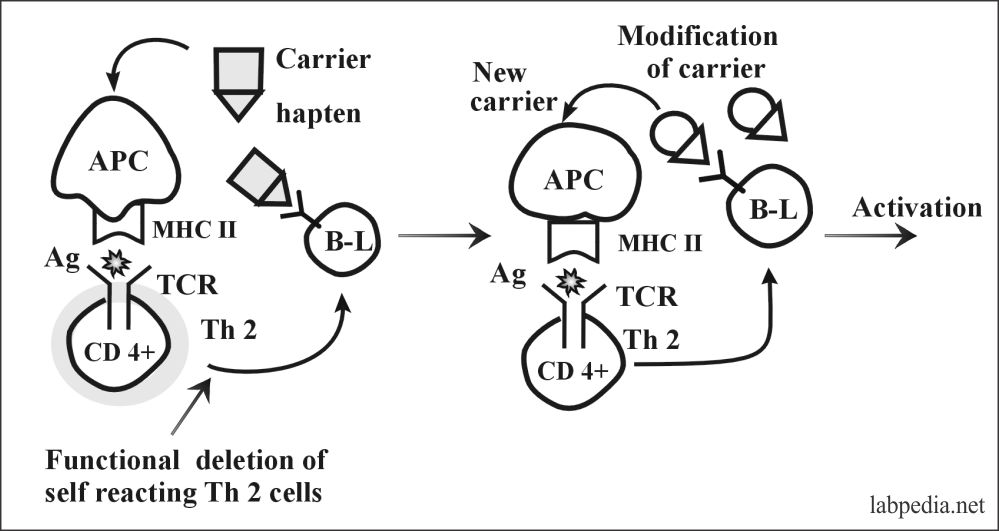
2. Bypass of Th (T-helper) Tolerance:
This can take place by modification of the molecular structure of antigen or carrier e.g.
- Modification of the molecular structure of potential autoantigen (Hapten) complexes with new carriers, e.g., α-methyldopa complex with e-antigen on RBC, and lead to autoimmune hemolytic anemia.
Partial degradation of auto-antigen is the exposure of new antigens like degraded collagen, thyroglobulin, and γ-globulin.
3. Cross-reaction (Molecular Mimicry)
Some of the microbes are a cross-reaction source, as like antibody against these antigens may cross-react with self-antigen. e.g., Rheumatic heart disease where antibody against the M-protein of streptococci reacts with the M-protein of the sarcolemma of cardiac muscle.
In ulcerative colitis–antigen E.coli 014 form an antibody which reacts with colon-polysaccharides.
4. Polyclonal Activation of B-Lymphocytes
There may be direct activation of B-L, and Th-cells may be bypassed.
This may take place by:
- Exogenous substances–lipopolysaccharides, Nystatin, PPD, and Amphotericin B.
- Endogenous or intrinsic substances.
- Microbes, e.g., viruses, bacteria, and parasites.
- Proteolytic enzyme-like trypsin.
- Viruses are a significant cause of B-lymphocytes’ polyclonal activation; they can lead to a Bypass of Th-cell.
- Direct stimulation of B-L.
- Loss of Ts.
The B-lymphocytes activation may be due to:
- Genetic abnormality.
- Intrinsic B-cell abnormality.
- Ts-function loss.
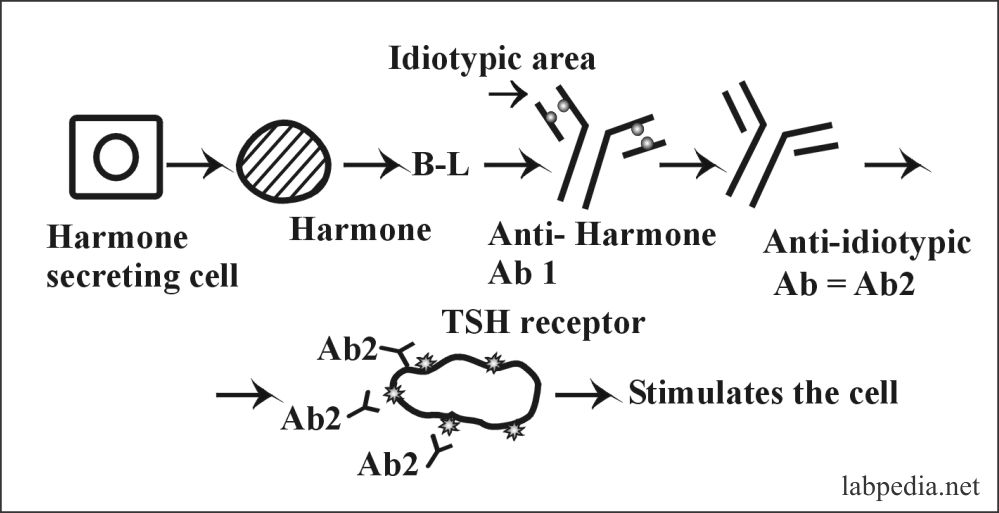
5. Idiotype-Bypass Mechanism
It is postulated that idiotype and anti-idiotype network regulate immune response, inhibiting or activating the response.
One of the examples is Grave’s disease, where auto-antibody against TSH-receptor stimulates the thyroid gland.
6. Imbalance of Ts-Th Function
Imbalance of Ts – Th cells is seen with increasing age. In SLE, it is found that there is an overactivity of the Helper cells.
7. The emergence of Sequestered Antigens
There are some autoantigens, which are not exposed to the immune system during developmental stages. These are exposed; autoantibodies form, e.g., spermatozoa, thyroglobulin, and eye lens.
In 1956–Dressler described autoantibodies formation after myocardial infarction where autoantibody forms against cardiac myocytes because of injury to these muscles. Here the function of the autoantibody is to clear the damaged tissue and recycle the affected protein.
Another mechanism is that virus may damage the tissue and expose the intracellular proteins. These autoantigens may incite an immune response.
The last possibility is that appearance of a small number of hidden autoantigens to which peripheral tolerance exists may be sufficient to break it since tolerance is dependent on the concentration of the antigens.
8. Thymic Defects
The intact thymus gland plays a vital role in immunologic stability because a thymectomy patient is more prone to develop autoimmune diseases.
9. Defects in Macrophages
As one knows, macrophagic cells are needed to process antigens and their presentation to immune cells. So any defect or abnormality of macrophagic cells may lead to autoimmune diseases.
10. Genetic Factors (Role of HLA)
There are reports of familial clustering of autoimmune diseases. There is a linkage of autoimmune disorders with MHC-II molecule e.g.
| HLA typing | Disease | Risk % |
| DR4 | Rheumatoid arthritis | 42 |
| DR2 and DR3 | Juvenile diabetes | 32 |
| B27 | Ankylosing spondylitis | 87.4 |
| DR3 | Grave’s disease | 37 |
| DR3 | Myasthenia Gravis | 2.5 |
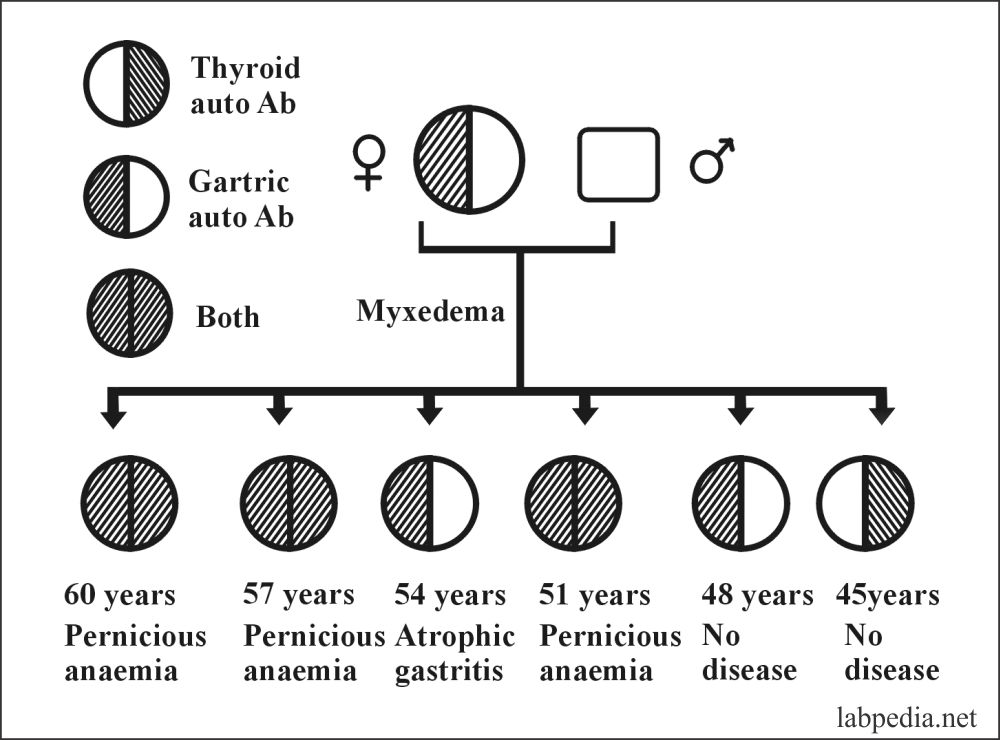
There is a famous history of autoimmune diseases in a family. Where the mother has myxedema, and all six children show evidence of autoimmune disease or autoantibody.
11. Cytokines
Cytokines are soluble factors that affect both locally and systemically cells of the immune system. These cytokines also play some role in the development of autoimmune disease e.g.
IL-2 is the T-lymphocyte growth and differentiation factor used therapeutically in some solid organs. Tumors to enhance immune-mediated anti-tumor responses. During such IL-2 treatment, an inflammatory lesion with lymphocytic infiltration has been seen in several organs, including myocardium, skin, liver, and thyroiditis, like Hashimoto’s, which may progress hypothyroidism in 10% of the cases.
IFN-α therapy: This is used to treat and clear hepatitis C virus infection. These patients may develop type-I diabetes with islets antibody and insulin autoantibody as evidence of autoimmune disease.
Summary
- Autoimmune diseases usually arise spontaneously.
- Infection plays a critical role in this process.
- Susceptibility to autoimmune disease is controlled by the environment and genetic factors (especially the MHC gene).
Therapy of Autoimmune Diseases
Various modalities may treat autoimmune diseases like:
- Replacement therapy for loss of endocrine secretions (insulin, thyroxin).
- “Blanket” suppression of the immune system by giving corticosteroids. This effect on all components of the immune system, e.g., interferes in cell activation and migration.
- Cyclosporine A and FK506-inhibit early events in T-cell activation. These inhibit cell-mediated immune response more selectively. These drugs have been used in clinical trials for the treatment of type I diabetes and rheumatoid arthritis.
- Monoclonal antibodies. It will block cell to cell interaction or neutralize positive signals such as cytokines.
Now we will discuss some of the autoimmune diseases. These are also called Rheumatic diseases or connective tissue, or collagen diseases.
Rheumatic means the migratory nature of the pain (Greek word) whiles the term connective tissue or collagen indicates the components typically affected.
Clinical manifestations center around the joints and muscles, but other systems are different diseases.