Calcium:- Part 2 – Ionized Calcium (Ca), Free Calcium
Ionized Calcium (Ca)
What sample is needed for Ionized Calcium?
- Collect blood anaerobically and draw it without applying pressure or causing stasis.
- The sample is stable for 6 hours at 4 °C.
- Plasma or serum can be stored for a longer period at -20 °C.
- The non-fasting sample is acceptable.
- The prolonged use of a tourniquet should be avoided because it lowers pH and increases calcium levels.
What are the indications for Ionized Calcium?
- This test determines physiologically active or free Calcium in patients with altered proteins, e.g, in chronic renal failure, nephrotic syndrome, malabsorption, and multiple myeloma.
- Ionized calcium values reflect calcium metabolism better than total calcium.
- A significant decrease in ionized calcium, regardless of total calcium, may lead to increased neuromuscular irritability and tetany.
- This is also recommended in cases of parathyroid disease.
- Patients with hypocalcemia or hypercalcemia have borderline serum calcium and altered serum proteins.
- Patients are receiving an intravenous blood transfusion.
- In case of major surgery.
- In case of abnormal blood proteins.
What are the precautions for calcium?
- Magnesium affects ionized calcium, so it is essential to measure magnesium levels in cases of hypocalcemia.
- A fasting specimen is preferred.
- The prolonged use of a tourniquet should be avoided because it lowers pH and increases calcium levels.
- Serum pH can affect the calcium level. A decreased pH can cause an increased level of calcium.
- Venous stasis or erect posture increased the calcium level by 0.6 mg/dL.
- Diurnal variation is higher in PM (about 9 PM) than in AM (lowest level).
- Separate immediately from RBCs to avoid calcium uptake by these cells (RBCs).
- Excessive milk intake leads to increased calcium levels.
- Vitamin D intoxication also increases the calcium level.
- Check the albumin level because hypoalbuminemia leads to an artificial decrease in the calcium level.
- Drugs like calcium salts, alkaline antacids, thiazide diuretics, vitamin D, parathyroid and thyroid hormones, and androgens may increase the serum calcium level.
- Drugs like aspirin, anticonvulsants, heparin, laxatives, diuretics, magnesium salts, and oral contraceptives may decrease the calcium level.
How will you define ionized calcium (Free calcium)?
- Ionized calcium (Free calcium) is the physiologically active form of calcium.
- The parathyroid glands, bone, kidneys, and intestine regulate ionized calcium homeostasis.
- There is a small diurnal change in ionized calcium, with the peak level occurring around 9 p.m. and the lowest level at 9 a.m.
- Ionized calcium level is not affected by changes in serum albumin.
What do you know about Ionized Calcium (free calcium)?
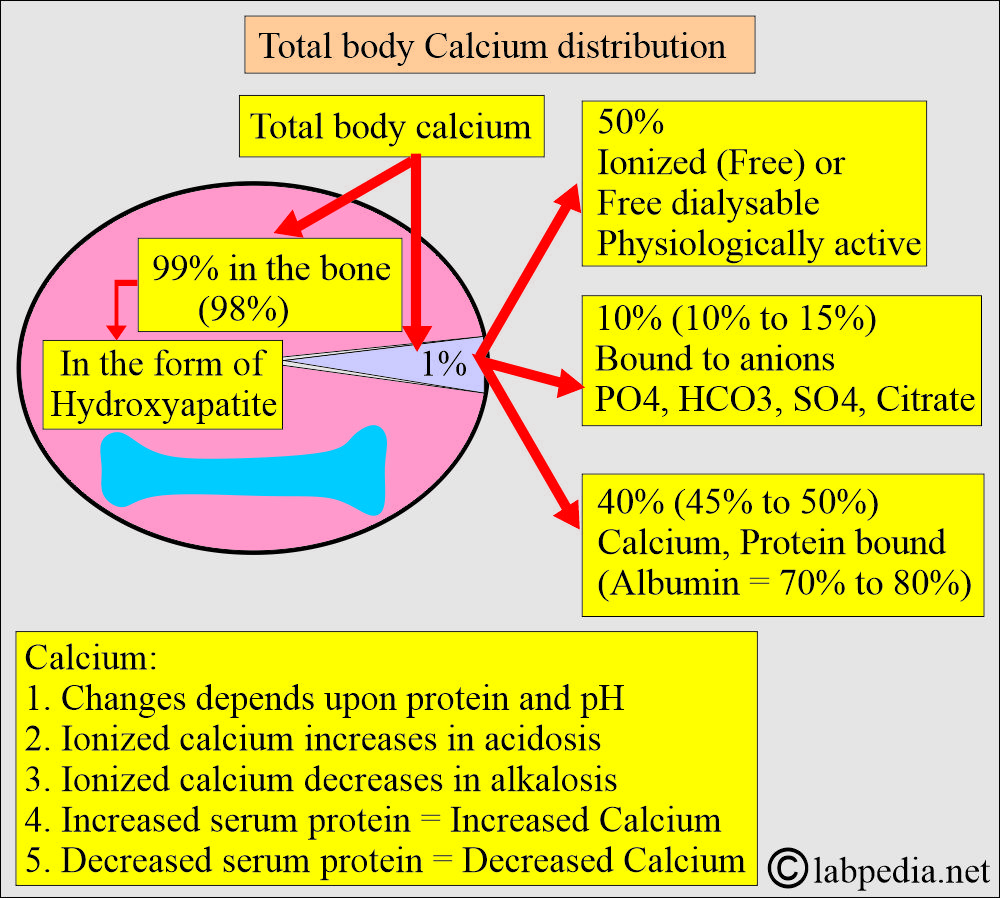
- The routine serum calcium measures the total serum calcium value.
- 99% of the calcium is present in the bones.
- It is present in soft tissue = 1%
- It is present in the extracellular fluid = 0.2%.
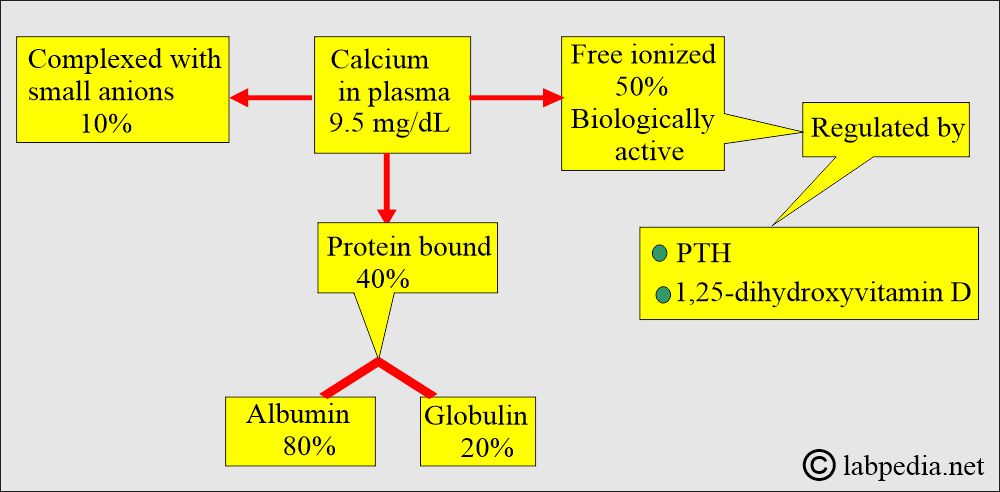
- Calcium in serum is:
- 50% Non-Bound calcium, also known as ionized or free dialyzable calcium. The free or ionized form is the active form.
- Only the ionized fraction is biologically active.
- 40% is bound to protein. The protein-bound fraction of calcium is bound to albumin (70% to 80%).
- 10% is complexed with non-protein compounds (ions), such as citrate, phosphate, sulfate, and bicarbonate.
- 50% Non-Bound calcium, also known as ionized or free dialyzable calcium. The free or ionized form is the active form.
- The most valuable clinical information is provided by a knowledge of the concentration of Free (ionized) Ca++ rather than of total Ca++ in primary hyperparathyroidism.
- In some patients with primary hyperparathyroidism, there is an increase in ionized calcium, but total calcium is normal.
- Ionized calcium (hypercalcemia) may be seen in:
- Multiple myeloma.
- Sarcoidosis.
- Hypervitaminosis D.
- Metastatic carcinoma infiltrates the bone.
- Most of the labs have no facility to measure ionized calcium.
- The ion-selective electrode can measure ionized calcium.
- There are formulas by which you can calculate the ionized calcium from the total serum calcium.
- In critically ill patients, an increased total serum calcium level usually indicates ionized hypercalcemia.
- Normal total serum calcium is evidence against ionized hypocalcemia.
- Ionized calcium is preferred over total serum calcium in some conditions:
- The liver transplantation.
- Rapid or large transfusion of citrated blood.
- In these conditions, the total serum calcium interpretation is almost impossible.
- The increase of ions to which calcium is bound is:
- Bicarbonate (HCO3).
- Citrate during a blood transfusion.
- Phosphate level. In the case of phosphate therapy in diabetic ketoacidosis, rhabdomyolysis, and chemotherapy (tumor lysis syndrome).
- Radiographic material containing calcium chelators.
What are the functions of Ionized calcium or free (dialysable) calcium?
- It exerts its physiological effects upon:
- The neuromuscular junction.
- Membranes.
- Bone deposition.
What is the normal Ionized Calcium (Free calcium)?
Source 1
- Ionized calcium:
- Whole blood adult = 4.65 to 5.28 mg/dL (1.175 to 1.375 mmol/L)
- Newborn = 4.20 to 5.58 mg/dL.
- The ratio of ionized Ca++ to total Ca++ = 48 to 56%.
Source 2
- Newborn = 4.20 to 5.58 mg/dL (1.05 to 1.37 mmol/L).
- 2 months to 18 years = 4.80 to 5.52 mg/dL (1.20 to 1.38 mmol/L).
- Adult = 4.5 to 5.6 mg/dL (1.05 to 1.3 mmol/L).
| Age | Whole blood mg/dL | Serum mg/dL | Capillary blood mg/dL |
|
|
||
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
||
|
|
||
|
|
||
|
|
- To convert into an SI unit, the multiplying factor is x 0.25 = mmol/L
How will you measure ionized calcium?
- It can be measured by the ion-selective electrode method.
- The above method is more accurate.
- A less accurate method to calculate serum calcium:
- Adjusted calcium = (Total serum calcium – serum albumin) + 4.0
- (Calcium in mg/dL and albumin in g/dL).
- Adjusted calcium = (Total serum calcium – serum albumin) + 4.0
- In SI unit formula = Adjusted calcium = (calcium – 0.025 albumin) + 1.0
- (Calcium in mmol/L and albumin in g/L).
- Ionized calcium values are affected by the blood pH level.
- A decrease of 0.1 pH = an increase in ionized calcium by 1.5% to 2.5%.
- If serum is exposed to air and stands for a long time.
What are the causes of an increased level of ionized calcium?
- Primary hyperparathyroidism. In 25% of patients, it is observed that total serum calcium is normal, while ionized calcium is elevated.
- PTH-producing tumors.
- Various malignancies.
- Excess intake of vitamin D.
- Acidosis.
- Neonatal total serum calcium associated with hypoalbuminemia may indicate ionized hypercalcemia.
What are the causes of a decreased level of ionized calcium?
- Primary hypoparathyroidism. It is also seen in secondary hypoparathyroidism.
- Alkalosis.
- Vitamin D deficiency.
- After a blood transfusion.
- After major surgery.
- Trauma, burns, and sepsis.
- Fat embolism.
- Pancreatitis.
- After hemodialysis.
- Multiple organ failures.
- Toxic shock syndrome.
- Increased calcium-binding to albumin in conditions like:
- Drugs like heparin, epinephrine, norepinephrine, alcohol, and isoproterenol.
- Acute pancreatitis.
- Diabetic ketoacidosis.
- Sepsis.
- AMI.
- Please see more details on the Calcium level (total) in part 1.
What is the Critical value of ionized calcium?
- Ionized calcium level <2 mg/dL (life-threatening).
- Ionized calcium level <3 mg/dL in multiple blood transfusions is the indication for calcium therapy.
What are the natural foods that are a good source of calcium?
| Food | Quantity | Amount of calcium |
| Kale | one cup | 245 mg |
| Milk | one cup | 305 mg |
| Yogurt | 6 oz | 300 mg |
| Cheese | one oz | 224 mg |
| Dried figs | 8 whole figs | 107 mg |
| White Beans | one cup | 191 mg |
| Turnip greens | one cup | 195 mg |
| Black-eyed beans | 1/2 cup | 185 mg |
| Canned salmon | 1/2 cup | 232 mg |
| Orange juice | one cup | 500 mg |
| Orange | one medium | 65 mg |
| Sesame seed | one teaspoon | 88 mg |
| Almond | 1/2 cup dry-roasted | 72 mg |
| Instant oatmeal | one cup | 187 mg |
| Soy milk | one cup | 300 mg |
| Firm Tofu | 1/2 cup | 861 mg |
| Broccoli | one cup | 62 mg |
Questions and answers:
Question 1: What is the significance of ionized calcium?
Question 2: What is the importance of ionized calcium estimation in the blood?