Calcium: – Part 1 – Calcium Total, Hypercalcemia and Hypocalcemia
Calcium Total
What sample is needed for Calcium Total?
- It is performed on the patient’s serum.
- The blood should be collected without much pressure on the arm.
- Avoid prolonged tourniquet.
- EDTA cannot be used as an anticoagulant for the plasma.
- Obtain blood with minimal venous occlusion, preferably without exercise, or after restoring circulation.
- The serum is stable for 8 hours at 22 to 25 °C. But it can be kept at 4 °C for a longer period.
What are the Indications of Calcium Total?
- The serum level of calcium is used to evaluate parathyroid function and metabolism.
- Serum calcium level is used to monitor renal failure and renal transplantation.
- Serum calcium level is used to evaluate hyperparathyroidism.
- Serum calcium levels may be assessed in malignancies such as multiple myeloma.
- Serum calcium levels may be monitored before and after blood transfusions.
- It is advised following thyroidectomy and parathyroidectomy.
- It is advised in acute pancreatitis.
- It is advisable to assess the effects of various drugs.
What are the Precautions for Calcium Total?
- Venous stasis during blood collection, caused by prolonged tourniquet application, increases the calcium level.
- A fasting specimen is preferred.
- Venous stasis or erect posture increased the calcium level by 0.6 mg/dL.
- Diurnal variation is higher in PM (around 9 PM) than in AM (lowest).
- Separate serum immediately from RBCs to avoid calcium uptake by these cells (RBCs).
- Excessive milk intake leads to increased calcium levels.
- Vitamin D intoxication also increases the calcium level.
- Check the albumin level because hypoalbuminemia leads to an artificial decrease in the calcium level.
- Drugs like calcium salts, alkaline antacids, thiazide diuretics, vitamin D, parathyroid and thyroid hormones, and androgens may increase the serum calcium level.
- Drugs like aspirin, anticonvulsants, heparin, laxatives, diuretics, magnesium salts, and oral contraceptives may decrease the calcium level.
- Calcium levels are increased by hyperalbuminemia, as seen in multiple myeloma and Waldenstrom macroglobulinemia.
- It is increased by dehydration.
- Hyponatremia (<120 meq/L) increases the protein-bound fraction of calcium. At the same time, hypernatremia decreases serum calcium.
- The hemodilution decreases serum calcium.
How will you define Calcium (Total)?
- Calcium exists in three forms:
- Physiologically active free calcium (Ionized calcium). It is around 50%.
- Protein-bound calcium, mostly bound to albumin. It is around 40%.
- Complexed with anions. It is around 10%.
- Calcium (Ca++) is our body’s 5th most common element and the most common cation in our body.
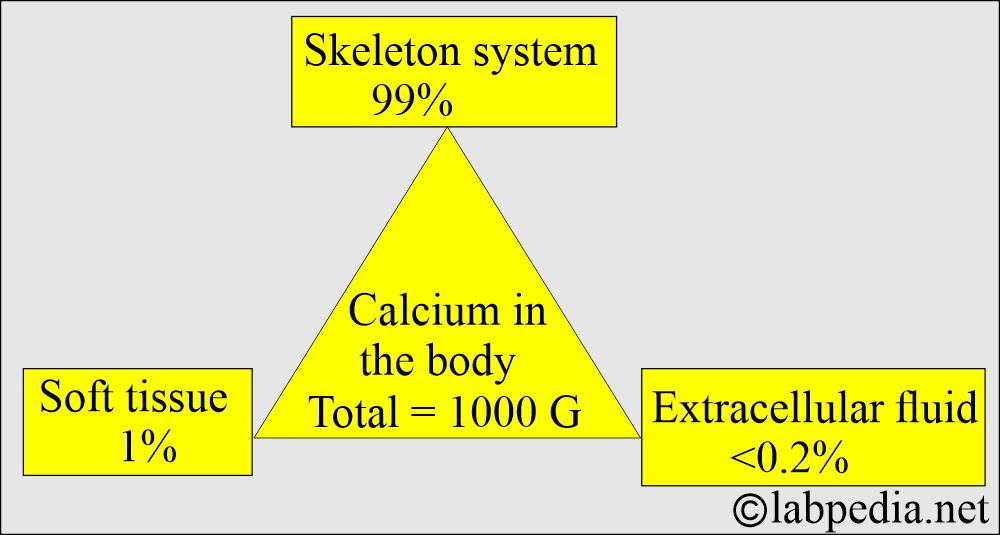
- The average human body contains approximately 1 kg (2.205 lb) of calcium, equivalent to 24.95 mol.
- Calcium is found in the skeleton, soft tissue, and extracellular fluid.
- The body requires a daily intake of approximately 400 mg of calcium.
- The minerals required by our body are:
- Sodium.
- Potassium.
- Calcium.
- Chloride.
- Phosphorus.
- Magnesium.
- Organically bound-S.
- Other elements required in trace amounts are:
- Iron.
- Zinc.
- Copper.
- Manganese.
- Selenium.
- Chromium.
- Molybdenum.
- Cobalt.
- Iodine.
How will you discuss the Calcium Metabolism?
Calcium distribution:
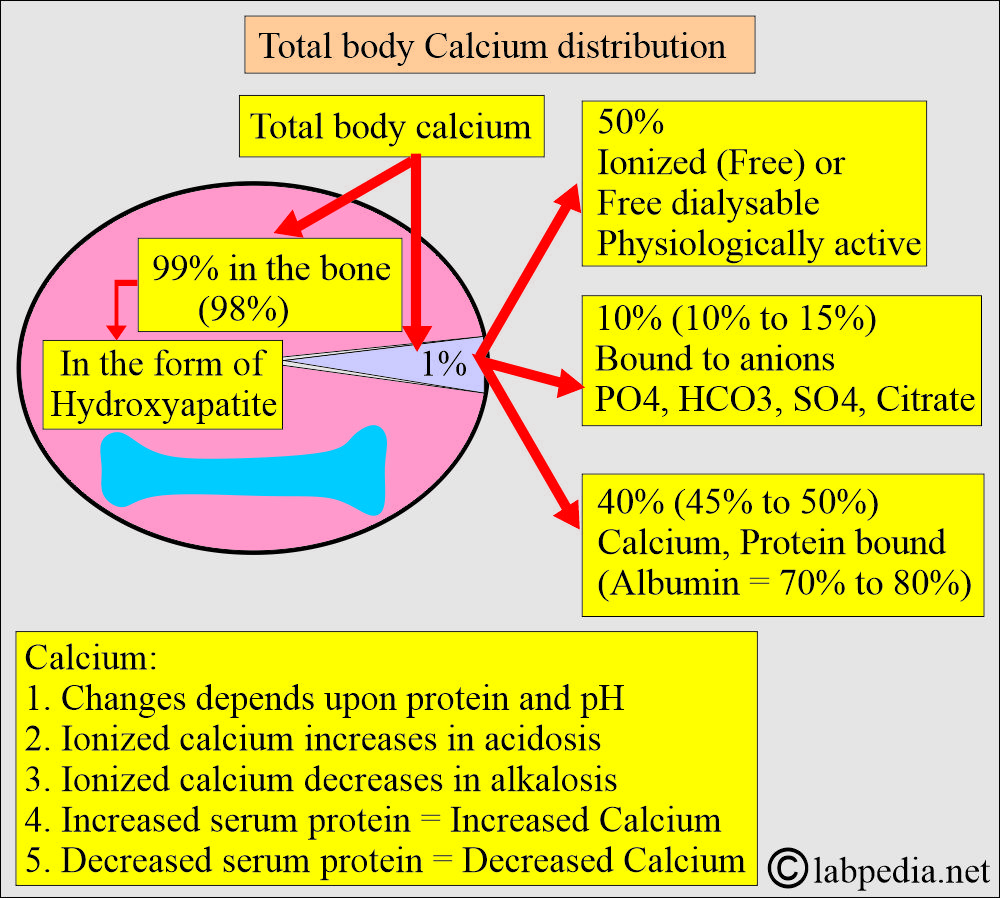
- There is a large amount of calcium in the body, mainly in the bones and teeth.
- About 99% of calcium is deposited in the skeleton as a mixture of:
- Amorphous calcium phosphate.
- Crystalline hydroxyapatite.
- Calcium phosphate crystal (hydrated).
- A small amount of fluoride is incorporated into the calcium phosphate in the teeth and bone.
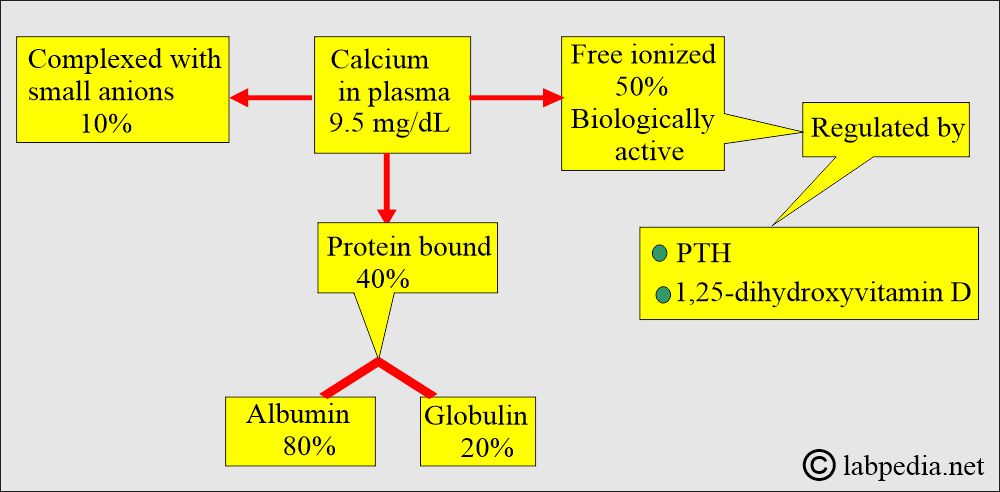
- Approximately half of the calcium in blood circulation is present in a free, ionized form, and the other half is protein-bound, primarily with albumin. Calcium is bound to protein as follows:
- 50% free or ionized (active) form of calcium.
- 40% is bound to the protein.
- 80% of calcium is bound to albumin, and 20% is bound to globulin.
- 10% is complex with anions.
- Some physician prefers ionized serum calcium levels to avoid the effect of albumin.
- Calcium is complexed with small diffusible anions:
- Bicarbonate.
- Lactate.
- Phosphate.
- Citrate.
- Calcium in the blood is virtually all present in the plasma.
- It increases in acidosis and decreases in alkalosis.
- An increase in the plasma proteins leads to an increase in serum total calcium.
- Decreased plasma proteins result in a decrease in total serum calcium.
- Approximately half of the calcium in blood circulation is present in a free, ionized form, while the other half is protein-bound, primarily with albumin.
What is the distribution of calcium in the body?
| Presence of Calcium in the body | Calcium amount | Phosphate amount |
|
|
|
|
|
|
|
|
|
|
|
|
What is the intake of calcium and balance (Absorption and excretion)?
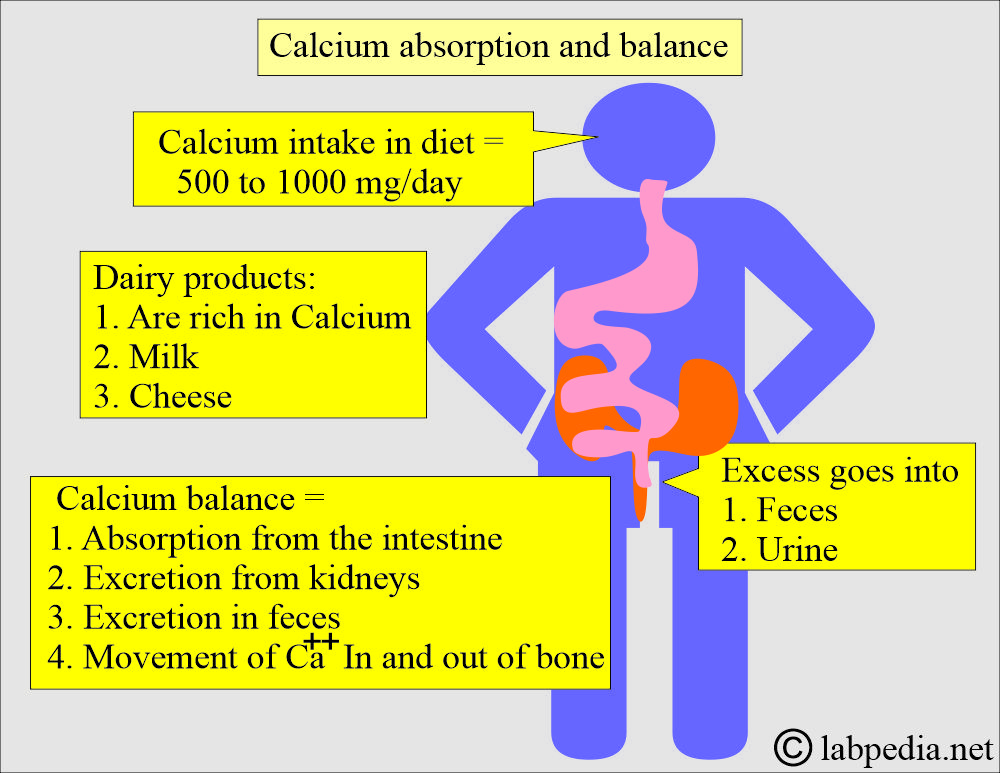
- Most individuals consume 500 to 1000 mg of calcium daily through their diet.
- They excrete excess amounts in the feces and urine.
- Dairy products, such as milk and cheese, are excellent sources of calcium.
- A large amount of dietary calcium is not absorbed because of the formation of insoluble calcium compounds like PO4, oxalate, phytate, and soap in the intestine and is excreted in the feces.
- Calcium balance is maintained by:
- Absorption of calcium from the intestine.
- Excretion by the kidneys.
- The movement of calcium in and out of the bones.
- Calcium phosphate in the bone is not an inert substance.
- There is dynamic equilibrium with Ca++ and HPO4– of the body fluids by resorption and deposition.
What are the functions of Calcium?
- Calcium is also essential for maintaining metabolic processes, including muscle contraction, the transmission of neural impulses, blood clotting, cardiac function, and inhibiting cell destruction.
- Calcium stabilizes the plasma membranes and influences permeability and excitability.
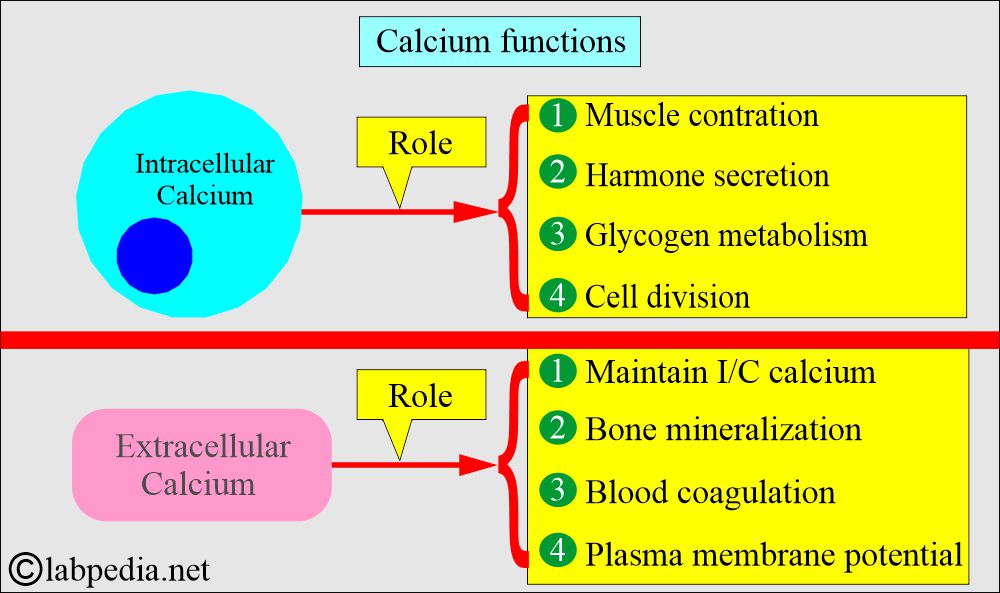
- The intracellular calcium functions are:
- Muscle contraction.
- Hormone secretion.
- Glycogen metabolism.
- Cell division.
- Activation of enzymes.
- Transfer of the ions across the cell membrane.
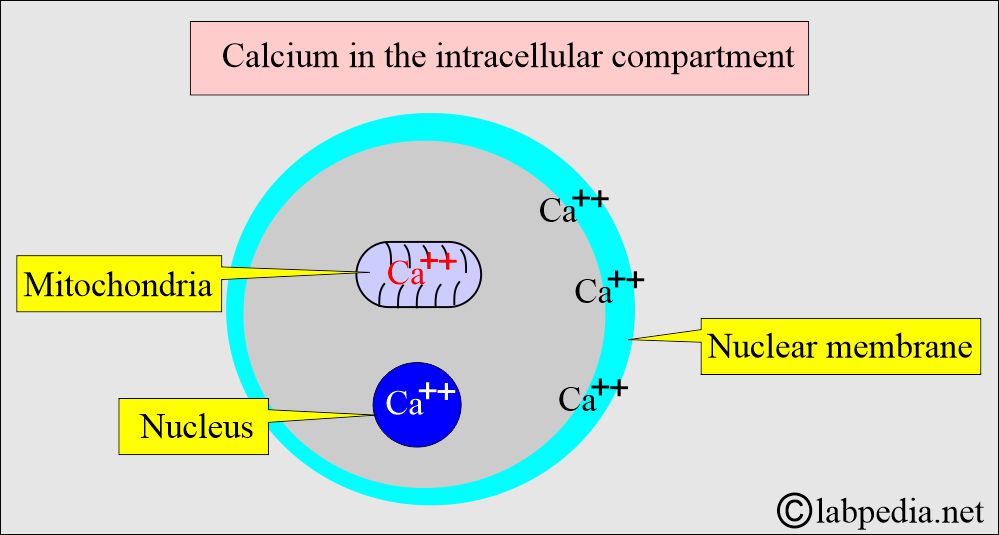
- The intracellular calcium is bound to:
- Protein in the cell membrane.
- Present in the mitochondria.
- Present in the nucleus.
- The extracellular calcium (extracellular calcium provides calcium ions) functions are:
- Bone Mineralization.
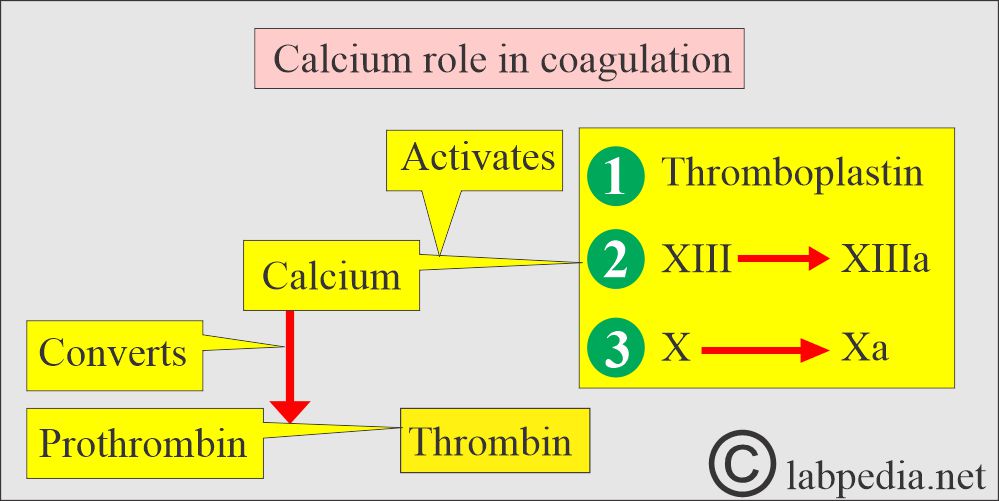
- Blood coagulation factors.
-
- Plasma membrane potential.
- Maintenance of intracellular calcium.
- Calcium decreases neuromuscular excitability.
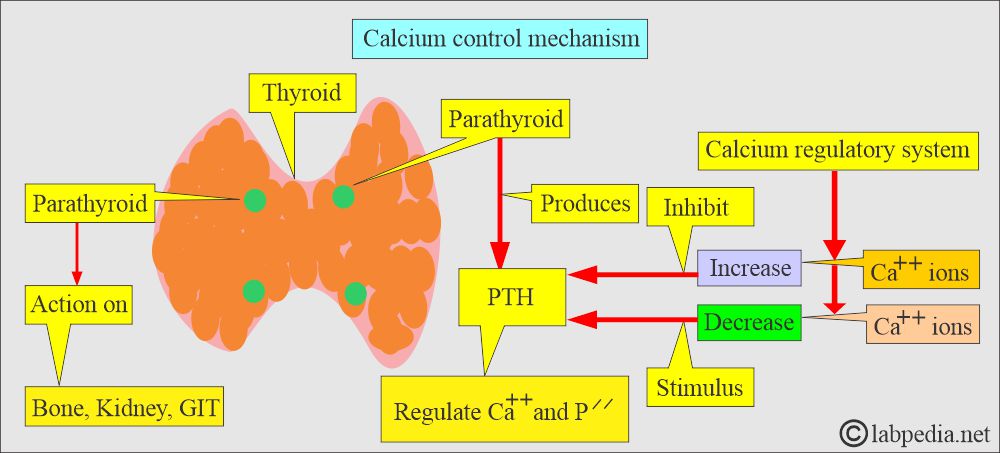
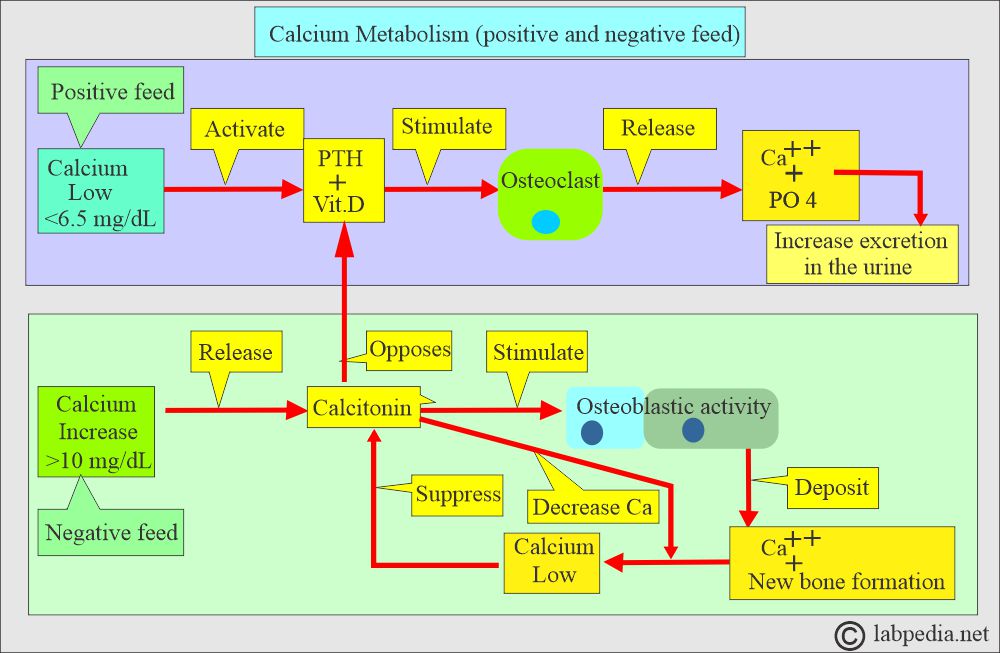
What is the Calcium control mechanism?
- Hypocalcemia:
- Normally, the level of calcium in the blood is carefully controlled. When blood calcium levels get low is called hypocalcemia.
- The bones release calcium to bring them back to a normal blood level.
- Hypercalcemia:
- When blood calcium levels become high, it is called Hypercalcemia.
- The extra calcium is stored in the bones or excreted through the stool and urine.
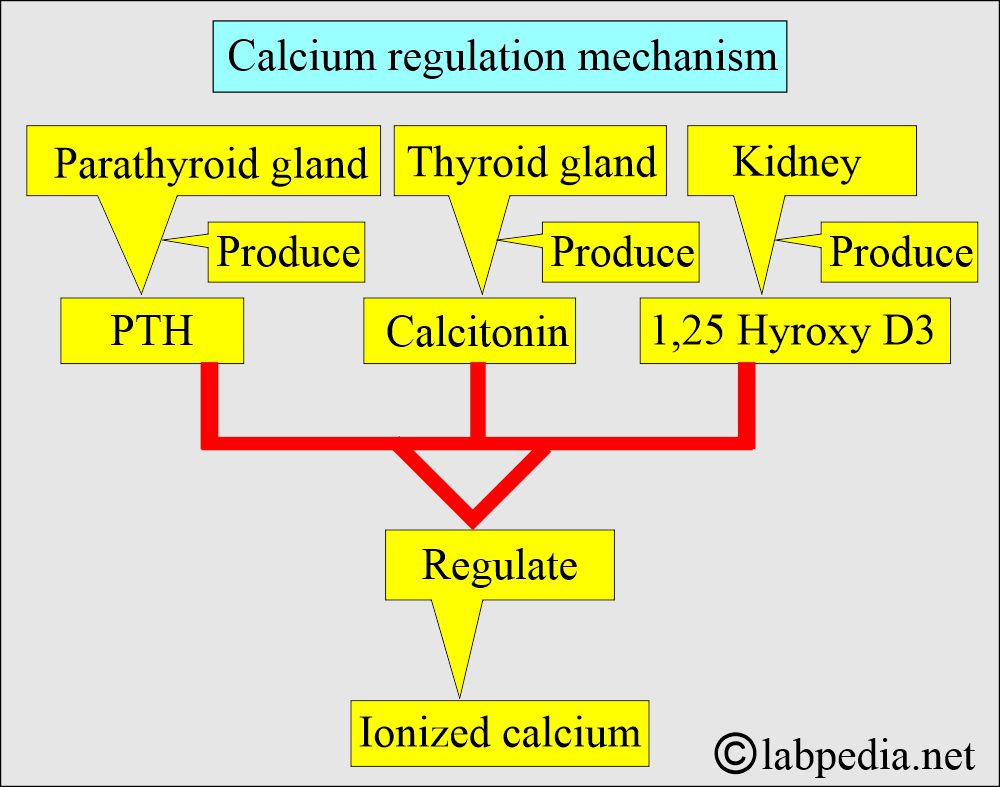
- Body serum calcium levels are maintained:
- By a parathyroid Hormone, PTH (Parathyroid gland hormone, calcium-regulating hormone).
- Calcitonin (produced by C or parafollicular thyroid cells) also plays a role in the control of serum calcium.
-
- The tumors of the lungs, breasts, and kidneys may secrete the ectopic PTH-like hormone.
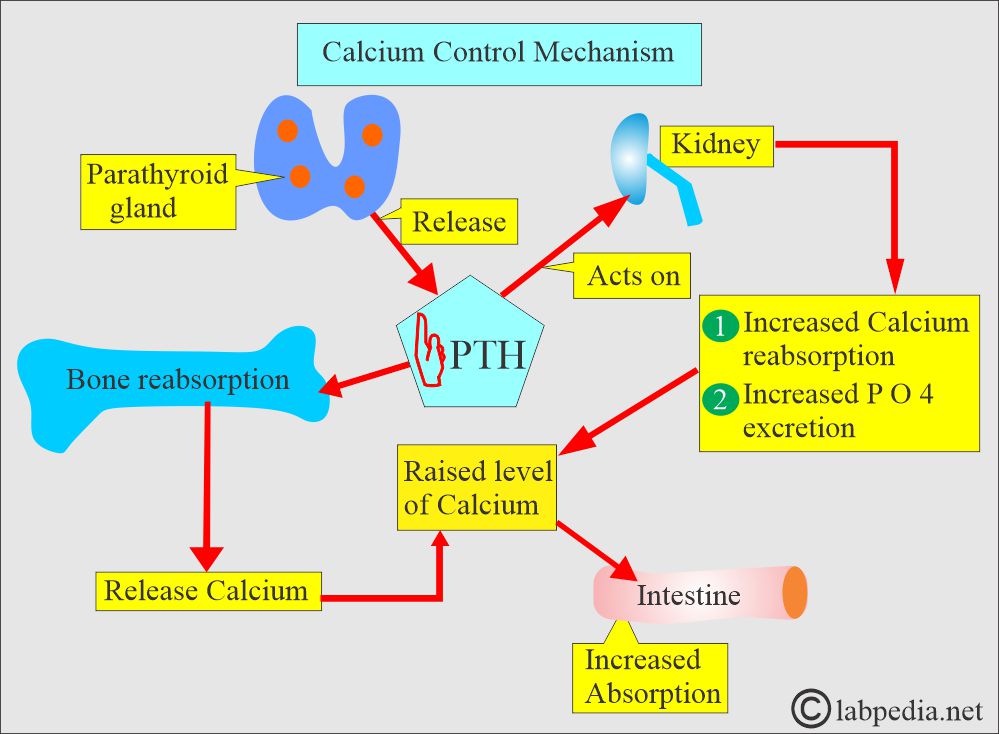
- PTH increases the serum calcium level:
- By increasing bone resorption.
- By mobilizing Calcium.
- PTH indirectly increases calcium absorption from the gastrointestinal tract by producing vit. D.
- PTH also increases the excretion of phosphate in the urine.
- Calcitonin decreases serum calcium and phosphate levels by inhibiting bone resorption.
- Decreasing calcium levels increases PTH, activating the calcium reservoir and releasing it into circulation.
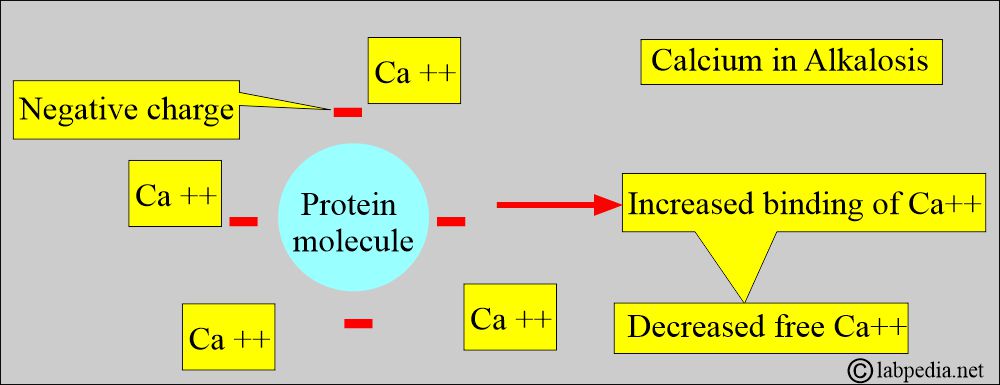
- Calcium binds to negative charge sites on the proteins, depending on the pH.
- Alkalosis, increased negative charge sites on proteins lead to increased calcium binding and decreased free calcium levels.
- In acidosis, the number of negative charge sites is decreased.
- H+ ions compete with calcium for binding sites on protein (mostly albumin), leading to an increase in free calcium.
- It will displace the calcium from albumin and increase the ionized or free calcium.
| Clinical condition | Total Calcium | Ionized or free calcium | Signs and symptoms |
|
|
|
May cause:
|
|
|
|
May cause:
|
- A patient with low serum albumin levels will typically have low serum calcium levels.
- Therefore, the serum albumin level can be estimated in conjunction with the serum calcium level. Serum calcium level decreases by 0.8 mg dL for every decrease of 1 g/dL of albumin.
What is the effect of the albumin on serum calcium?
- A decrease in 1 gram/dL of serum albumin decreases total serum calcium by around 0.8 mg/dL.
- This represents the average decrease, but it may vary in different situations.
- Because no effect of serum albumin on ionized calcium is preferred.
Hypocalcemia:
What is the etiology of hypocalcemia?
- The most common cause is hypoalbuminemia.
- One gram/dL of albumin binds 0.8 milligrams per deciliter of calcium.
- Therefore, there may be a decrease in albumin-bound calcium.
- Or a decrease in free calcium.
- Chronic renal failure leads to hypocalcemia because:
- There is an increased loss of protein in the urine due to kidney damage.
- There is hyperphosphatemia.
- There is a decrease in serum 1,25(OH) 2D.
- There is skeletal resistance to PTH.
What are the signs and symptoms of hypocalcemia?
- Neuromuscular hyperexcitability.
- Like tetany.
- Paresthesia.
- Seizures.
- A rapid fall in calcium level may lead to hypotension.
- Acute symptomatic hypocalcemia may be associated with the following:
- Rapid remineralization of the bone after the surgery for primary hyperparathyroidism is called hungry bone syndrome.
- Acute pancreatitis.
Hypercalcemia:
What is the etiology of hypercalcemia?
- Hypercalcemia is due to an increase in the influx of calcium into the extracellular compartment from:
- Skeletal system.
- Intestine.
- Kidney.
- Primary hyperparathyroidism is the most common cause in outdoor patients.
- Malignancy is the most common cause of hospitalized patients.
- The above two conditions constitute 90% to 95% of the causes of hypercalcemia.
- Also seen in 10% to 20% of cancer cases.
- Hypercalcemia (malignancy-associated) is seen in the following cancers:
- Myeloma = 30% (20% to 50%).
- Renal cell carcinoma = 11% (10% to 13%) due to ectopic production of PTH.
- Lung cancers = 10% (7% to 13%) due to ectopic production of PTH.
- Breast = 15% 7% to 23% due to ectopic production of PTH.
- Non-Hodgkins lymphoma = 5% (3% to 13%).
- Leukemia = 3% (2% to 11%).
- Some lymphomas may produce 1,25(OH)2 D and cause hypercalcemia.
- Primary sarcoidosis is associated with hypercalcemia in 5% to 10% of cases (ranging from 1% to 62%).
What are the signs and symptoms of hypercalcemia?
- Anorexia.
- Lethargy.
- Nausea.
- Vomiting.
- Ultimately coma.
- Other patients may show:
- Nervousness.
- Excitability.
- Tetany.
What are the criteria to label hypercalcemia?
- Three separate raised levels of calcium.
- Serum or plasma calcium = >10.3 mg/dL
- Ionized calcium = >1.30 mmol/L.
What are the normal Calcium levels?
Source 1
- Infant to one month = 7.0 to 11.5 mg/dL.
- One month to one year = 8.6 to 10.2 mg/ dL.
- Adult = 9 to 10.5 mg/dL.
- Its low level may lead to tetany.
Source 2
| Age | mg/dL |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- The conversion factor is x 0.25 for SI unit mmol/L
What are the causes of Hypercalcemia when the plasma level = >10.5 mg/dL?
- Hyperparathyroidism (primary and secondary).
- Hyperthyroidism.
- Acute and chronic renal failure.
- Following a renal transplant.
- Metastatic bone tumor of the lung, breast, and kidney.
- Bone metastasis occurs in around 30% of cases.
- 2% of Hodgkin’s lymphoma and non-Hodgkin’s lymphoma.
- Milk-alkali syndrome.
- Multiple myelomas.
- Paget’s disease.
- Osteomalacia with malabsorption.
- Aluminum-associated osteomalacia.
- Granulomatous diseases:
- Sarcoidosis (mostly uncommon).
- Tuberculosis.
- Leprosy.
- Uncommonly, it may be seen in:
- Mycosis.
- Berylliosis.
- Silicon granuloma.
- Crohn’s disease.
- Eosinophilic granuloma.
- Tumors produce a PTH-like substance.
- Vitamin D intoxication.
- Ectopic production of 1,25 -dihydroxy -vitamin-D3, e.g., from Hodgkin’s and Non-Hodgkin’s lymphoma.
- Vitamin A intoxication.
- Excessive calcium intake.
- Prolonged immobilization.
- Thiazide diuretics.
- Withdrawal of steroids.
- Most (80 to 90 %) of hypercalcemia cases are due to hyperparathyroidism or malignancy.
- Thyrotoxicosis in 20% to 40% of the cases is usually <14 mg/dL.
- Multiple endocrine neoplasias.
- Hypercalcemia is also seen in some of the neoplasms:
- Breast = 15% (7 to 23%).
- Lung = 10% (7 to 13%).
- Kidneys = 11% (10.5 to 13%).
- Colon = 5%.
- Cervix = 7%
- Non-Hodgkin’s lymphoma = 5% (3 to 13%).
- Leukemia = 3% (2 to 11.5%)
- Multiple myeloma = 30% (20 to 50%).
Causes of Hypercalcemia (>10.5 mg/dL):
| Clinical condition | Etiological causes |
|
|
|
|
|
|
|
|
|
|
|
|
What are the causes of Hypocalcemia (plasma level = <8.5 mg/dL)?
- Hypoparathyroidism.
- Surgical.
- Idiopathic.
- Infiltration of the parathyroid gland by sarcoidosis, amyloidosis, hemochromatosis, and neoplasm.
- Hereditary conditions like DiGeorge syndrome.
- Pseudohypoparathyroidism is due to a lack of response to PTH.
- Malabsorption (inadequate absorption of nutrients from the intestinal tract).
- Hypoalbuminemia.
- Osteomalacia
- Pancreatitis
- Renal failure (chronic). Chronic renal failure with uremia and phosphate retention.
- It is also seen in Fanconi syndrome and renal tubular acidosis.
- Bone diseases like osteomalacia and rickets.
- Malabsorption of calcium and vitamin D.
- Insufficient ingestion of calcium, phosphorus, and vitamin D.
- Starvation.
- Liver disease (decreased albumin production).
- Obstructive jaundice.
- Hypomagnesemia.
- Hyperphosphatemia, such as phosphate enema and infusion.
- Drugs like:
- Cancer chemotherapy, such as cisplatin, cytosine arabinoside, and mithramycin.
- Antibiotics like ketoconazole, gentamicin, and pentamidine.
- Chronic use of anticonvulsant drugs like phenobarbitol and phenytoin.
- Use of calcitonin.
- Excessive use of fluoride (fluoride intoxication).
- Neonates of complicated pregnancies.
- Infants of diabetic mothers.
- Premature babies.
- In the case of osteoblastic tumor metastasis.
- In the case of cerebral injuries.
- Temporary hypocalcemia after subtotal thyroidectomy was seen in >40% of the patients, and >20% showed signs and symptoms.
What are the Lab findings of hypocalcemia?
| Clinical conditions of hypocalcemia | PTH level | Serum PO4 | 1,25-Hydroxy-D |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What are the causes of hypocalcemia?
| Lab parameters | Causes of the increase in lab findings | Causes of the decrease in the lab findings |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What is the most common cause of hypocalcemia?
- It may be seen in Hypoalbuminemia, in which the ionized fraction may be normal while the total calcium level is decreased due to the low percentage of calcium bound to albumin.
- A correction formula is needed as follows:
- Corrected calcium level = measured calcium — albumin g/dL + 4
What are the natural food sources for calcium?
| Food | Quantity | Amount of calcium |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What are the Critical values of Calcium?
- Acute hypo or hypercalcemia can be life-threatening
- > 14 mg/dL Hypercalcemia leads to coma and cardiac arrest.
- < 4 mg/dL Hypocalcemia leads to tetany (another reference says <6.5 mg/dL).
Questions and answers:
Question 1: What is the calcium level which leads to tetany?
Question 2: What is the cause of hypercalcemia in renal cell carcinoma?