Anemia:- Part 8 – Hemolytic Anemias Classification, Lab Diagnosis
Hemolytic Anemias
Sample for Hemolytic Anemias
- Make a fresh blood smear.
- Take blood in EDTA.
- Bone marrow may be advised if needed.
Definition of hemolytic anemias
- This is a hemolytic state when the RBC’s life in vivo is shortened.
- The presence of anemia depends upon the degree of hemolysis and the response of the bone marrow.
- Hemolytic anemia results from the premature destruction of the peripheral blood RBCs.
The normal removal of the RBCs:
- RBC destruction occurs after a mean life span of 120 days when the MN phagocytic system removes these in the extravascular space, especially in the bone marrow, spleen, and liver.
The site of hemolysis is:
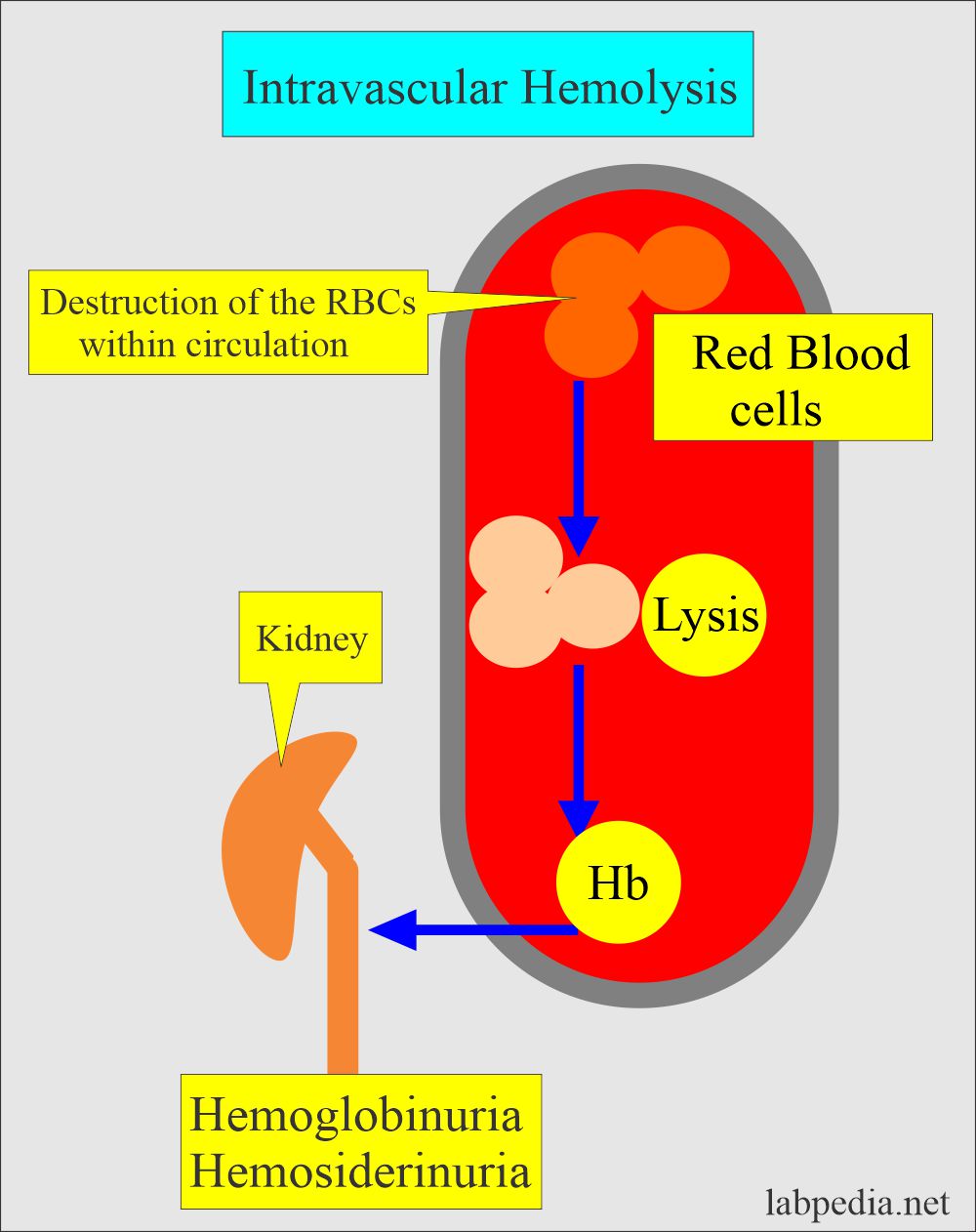
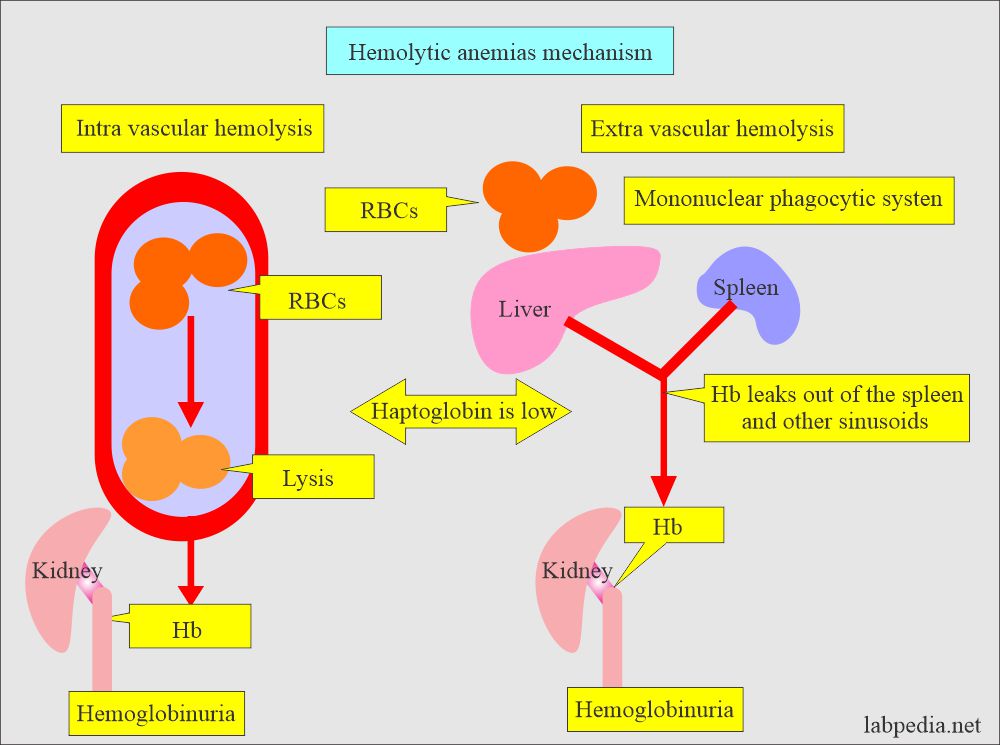
- Intravascular hemolysis occurs when the RBCs are destroyed in the circulation (blood vessels).
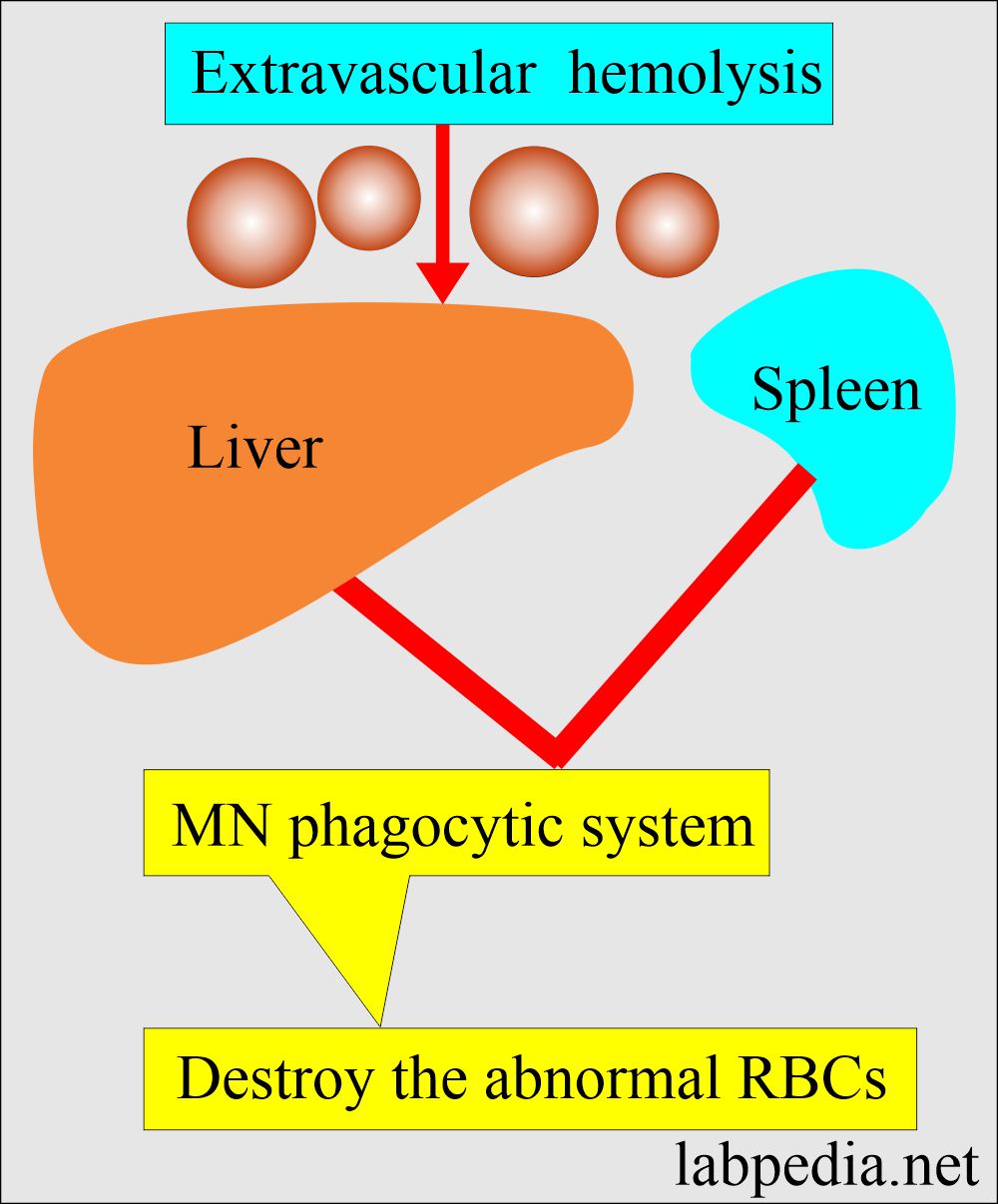
- Extravascular hemolysis occurs when the RBCs are destroyed by the mononuclear phagocytic system of the spleen, liver, and bone marrow.
Hemolytic anemias mechanism:
- These are defined as when there is increased destruction of the red blood cells.
- Because the compensation from the bone marrow anemia may not appear, when the destruction of RBCs becomes several times more than this compensatory mechanism, it will lead to hemolytic anemia.
- Hemolytic Anemia may be:
- Intrinsic factors where the RBCs are abnormal.
- Extrinsic factors when the external factors will destroy the RBCs.
- The warm antibody reacts in-vitro best at 37°C.
- The cold antibody reacts at 4°C to 10°C.
- Hemolytic anemias may be due to the following:
- Congenital.
- Acquired defects.
- All these anemias share common defects, which are shortened survival of the RBCs.
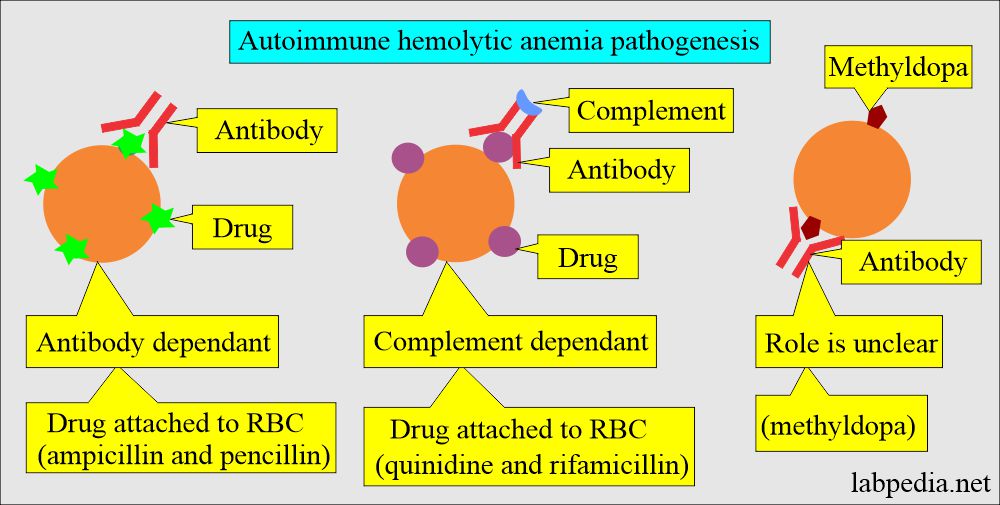
Role of drugs and antibodies:
- Combining the drugs with anti-drug antibodies forms the immune complex that is adsorbed onto the RBCs, often activating the complement.
- The antiquinidine antibody is IgM.
- The drugs bind to the RBCs membrane and act as the haptens.
- Penicillin is the best example. There is the development of this type of reaction in a few cases around 3%.
- There may be a nonspecific coating of the RBCs by drugs and various proteins.
- Antibiotics like Cephalothin act on this principle.
- The unknown mechanism where α-methyldopa leads to hemolytic anemia.
- The antibody against the α-methyldopa is IgG, which usually has Rh group specificity.
Traumatic microangiopathic hemolytic anemia.
- There are conditions that produce hemolytic anemia, forming many schistocytes, which may form due to trauma.
- The common examples are:
- Disseminated intravascular coagulation (DIC).
- Thrombotic thrombocytopenic purpura.
- The hemolytic uremic syndrome.
- Cardiac prosthesis syndrome.
- Long-term indwelling catheters.
- Hemolytic anemia is associated with vascular graft.
- In gastric carcinomas.
- In Clostridium welchii septicemia.
- Paroxysmal nocturnal hemoglobinuria (PNH).
- This is an acquired blood cell membrane defect in which RBCs, WBCs, and platelets demonstrate abnormal sensitivity to the effect of activated serum complement.
- These are manifested by hemolytic anemia, leucopenia, and thrombocytopenia.
- PNH affects young or middle-aged adults with the usual age range of 10 to 60 years.
- Abdominal pain in about 10% of the cases and hemoglobinuria in 50% of the cases.
- Anemia is usually of a moderate degree except during the acute episode of crises when it is severe.
- Hemolytic anemia due to toxins.
- Lead poisoning is the most frequent cause.
- This is usually seen in the ingestion of paint, which is frequent in children.
- Hemolytic anemia due to bacteria.
- This is usually seen in septicemia due to Clostridium welchii.
- This may produce severe hemolytic anemia with the presence of spherocytes.
- Hemolytic anemia due to parasitic infestation.
- The most frequent cause is malaria.
- Bartonella infection may cause hemolytic anemia.
- This is most common in Peru.
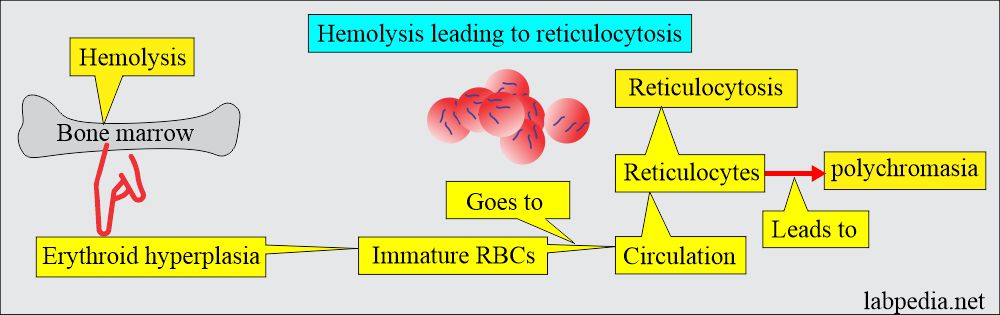
The outcome of the hemolysis:
- If the bone marrow hyperplasia compensates for the RBC loss, then no anemia will develop, which is called compensated hemolytic disease.
- The bone marrow becomes hyperplastic, and the increase in erythropoiesis increases 6 to 8 times the normal. Also, there is an increase in the volume of active marrow.
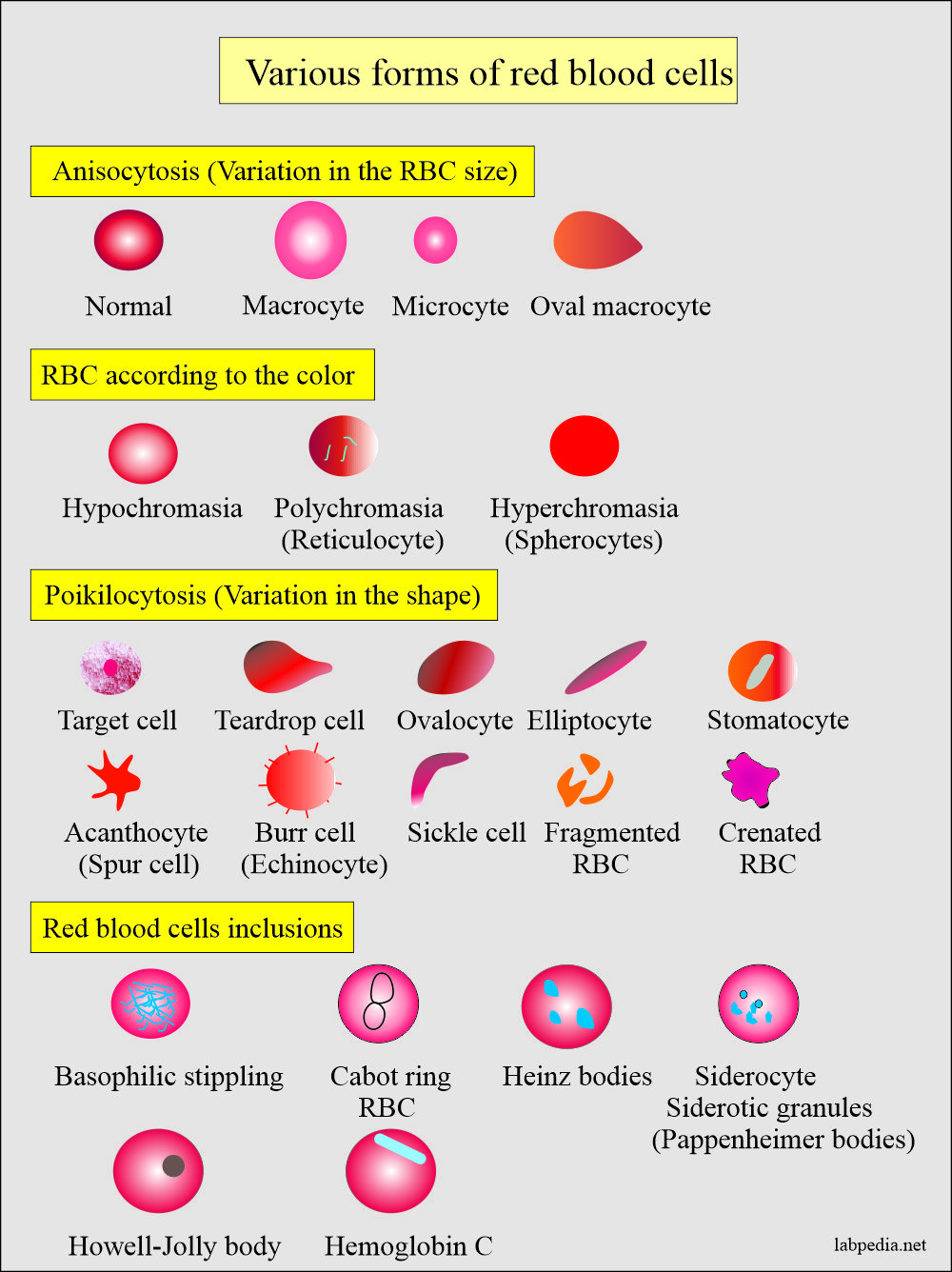
- Immature RBCs (reticulocytes) are released prematurely into the circulation and give rise to polychromasia.
Classification of hemolytic anemia:
- Anemia due to isoagglutinin (isoantibodies).
- These are caused by the antibodies within blood group systems.
- Anemia due to autoagglutinins (autoimmune hemolytic anemia or acquired hemolytic anemia):
- Antibodies are produced against self-own body tissue.
- Warm autoantibodies. These are IgG and are usually directed against Rh-antigen.
- These 50% to 70% are Coomb’s positive autoantibodies.
- Cold autoantibodies. These are IgM antibodies usually directed against the I antigen on the RBC membranes.
- These are 15% to 30% of Coomb’s positive autoantibodies.
- Warm autoantibodies. These are IgG and are usually directed against Rh-antigen.
- Antibodies are produced against self-own body tissue.
- Paroxysmal cold hemoglobinuria (PCH).
- This is a rare syndrome.
- The antibodies of the IgG class binds to and sensitizes RBCs at cold temperature.
- It produces complement-activated RBCs hemolysis at warmer temperatures.
- PCH is 2% to 5% of Coombs-positive autoantibodies.
- These are more common in children than in adults.
- Hemoglobinuria is produced after exposure to cold.
- Secondary acquired autoimmune hemolytic anemia.
- This may be a cold or warm variety. This can be divided into 3 main groups.
- Due to leukemia or lymphoma.
- Most often is due to chronic lymphocytic leukemia.
- Less frequent lymphocytic lymphoma.
- Rarely Hodgkin’s lymphoma.
- Due to collagen diseases like SLE (systemic lupus erythematosus).
- Miscellaneous systemic diseases like viral infections, severe liver diseases, ovarian tumors, and carcinomatosis.
- Due to leukemia or lymphoma.
- This may be a cold or warm variety. This can be divided into 3 main groups.
- Drug-induced hemolytic anemia.
- Sometimes drug-induced hemolytic anemia is included in autoimmune hemolytic anemia.
- Antibodies are formed against the drugs and the action on the RBCs is secondary due to the presence of drugs present on the surface of RBCs.
- There are 10% to 20% of Coombs-positive autoantibodies.
Hemolytic Anemias classification based on Coomb’s test:
| Coomb’s positive hemolytic anemia | Coomb’s negative hemolytic anemia |
Antibody-dependant hemolysis:
|
Non-antibody dependant hemolysis: Intrinsic RBCs abnormalities:
Extrinsic RBCs mechanism:
|
The etiology of RBC hemolysis may be:
- Hereditary is due to intrinsic red cell defects.
- Acquired are due to environmental changes.
- Hemolytic anemia can also be divided into:
- Anemias associated with intrinsic/intracorpuscular defects of RBCs.
- The life of the RBCs in patients and even in the recipient is shortened.
- Anemias associated with extrinsic/extracorpuscular defects of RBCs.
- In these cases, if transfuse normal RBCs in this group will be destroyed more rapidly.
- Anemias associated with intrinsic/intracorpuscular defects of RBCs.
- Hemolytic anemia can also be divided into:
- Intravascular hemolysis:
- It occurs when the destruction of the RBCs takes place in the blood vessels and hemoglobin is released.
- This is associated with physical trauma, like an artificial heart valve.
- Or this is associated with the complement system like paroxysmal nocturnal hemoglobinuria.
- Hemoglobin in the blood gives rise to hemoglobinuria.
- Causes are:
- Mismatched blood transfusion.
- G6PD deficiency is associated with stress.
- March hemoglobinuria.
- Few autoimmune hemolytic anemias.
- Extravascular hemolysis:
- When the destruction of the RBCs takes place in the mononuclear phagocytic system.
- The MNphagocytic system will trap and disrupt the abnormal cells.
- The spleen is very efficient in this respect and is followed by the liver.
- That is why there is splenomegaly in hemolytic anemia (disease).
- Abnormal hemoglobin:
- The hemoglobin function abnormalities and Heinz body formation cause the RBCs to lose their elasticity. When such RBCs go through the microcirculation, the RBC membrane cannot adjust to the small blood vessels, which are trapped and destroyed.
Hereditary Causes of hemolytic anemia:
- RBC cell membrane defect:
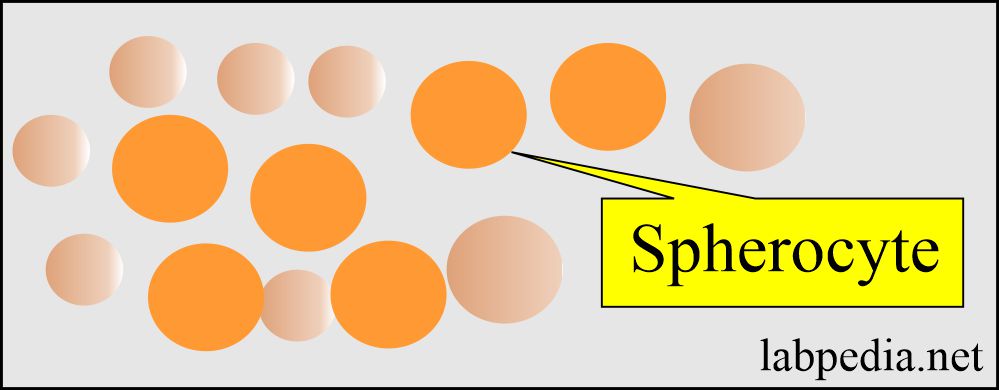
- Abnormality in the cell membrane cytoskeleton leads to spherocytosis and elliptocytosis.
- Abnormal lipid synthesis is like an increase in the membrane lecithin.
- RBC cell enzyme deficiency:
- G-6-PD deficiency.
- Pyruvate kinase deficiency.
- Hexokinase deficiency
- Glutathione synthetase deficiency
- Disorders of hemoglobin synthesis:
- Hemoglobin synthesis defects like Thalassemia.
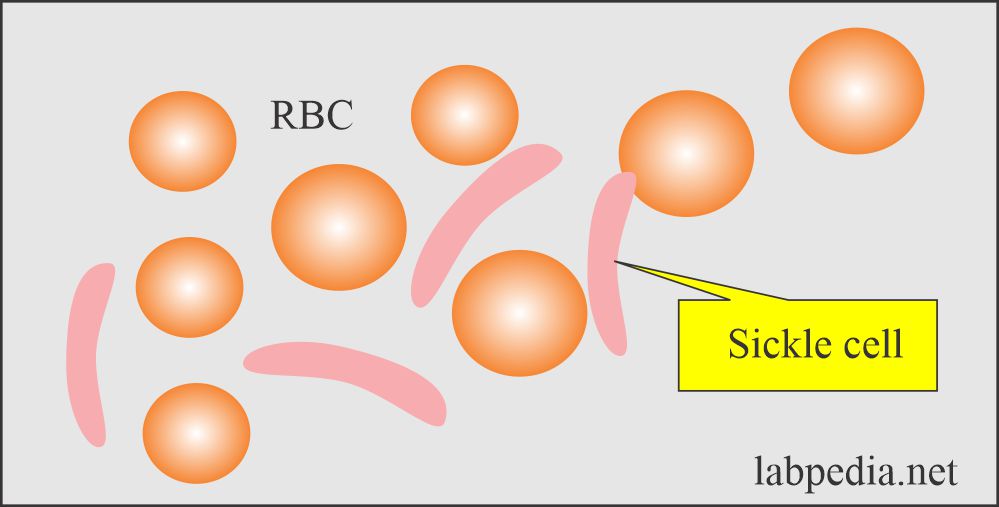
- Abnormal structure of the hemoglobin-like Sickle cell anemia, Hb E.
Acquired Causes of hemolytic anemia:
- Antibody-mediated cytotoxic reaction (Type II) like ABO incompatibility.
- Hemolytic autoimmune anemia is seen with cold antibodies and warm antibodies.
- Drug-induced hemolytic anemia is seen in Methyldopa, penicillin,
- Microangiopathic hemolytic anemia is seen due to RBCs’ trauma in the cardiac prosthetic valve and fibrin deposition in the microvasculature.
- Infections: Hemolytic anemia due to toxins like a malarial parasite, septicemia, Clostridium Welchii, pneumococci, staphylococci, and lead poisoning.
- Hemolytic anemia due to snake venom and spider bite.
- Hemolytic anemia due to lead poisoning and copper toxicity.
- Hemolytic anemia due to extensive burns.
- Hemolytic anemia in splenomegaly.
- Paroxysmal nocturnal hemoglobinuria.
- Anemia due to acquired RBC membrane abnormality.
- Secondary to liver and kidney diseases.
Causes of hemolytic anemia:
| Congenital causes of hemolytic anemia (inherited) | Acquired causes of hemolytic anemia |
|
|
Another classification of hemolytic anemia:
| Intracorpuscular causes | Extracorpuscular causes |
|
|
|
Clinical presentation of hemolytic anemia:
- There is pallor of the mucous membrane, which can be judged from the tongue.
- There is fluctuating jaundice. In some patients, bilirubin will be very high and may need blood transfusion exchange.
- Urine shows no bilirubin, but there is the presence of the urobilinogen.
- Gallstones are common in these patients.
- Patients with sickle cell disease may develop ulcers around the ankle.
- Aplastic crises may be seen in these patients.
- Rarely does folate deficiency cause aplastic crises.
Autoimmune hemolytic anemia:
Definition of Autoimmune hemolytic anemia:
- These are caused by the production of the antibody against self-RBCs.
- These are characterized by the direct Coomb’s test (direct antiglobulin test) and divided into:
A: Warm antibody Autoimmune Hemolytic anemia:
- Reacts more strongly at 37 °C.
- The type of antibody is IgG.
- Coomb’s test is strongly positive.
- Mostly the cause is idiopathic.
- This is an autoimmune phenomenon, and the best example is SLE, lymphoma, Hodgkin’s disease, and drugs (methyldopa).
- Signs and symptoms:
- 30% of the cases’ cause is not known.
- These anemias may occur at all ages and in both sexes.
- But more common in middle-aged females.
- There attack of relapse and remission with a short period of anemia and jaundice.
- This may progress to the chronic form.
- It is usually splenomegaly.
- Infection or folate deficiency may lead to a falling level of hemoglobin.
- Diagnosis:
- There is reticulocytosis.
- Bone marrow shows erythroid hyperplasia.
- Raised serum bilirubin level.
- Increased urinary urobilinogen.
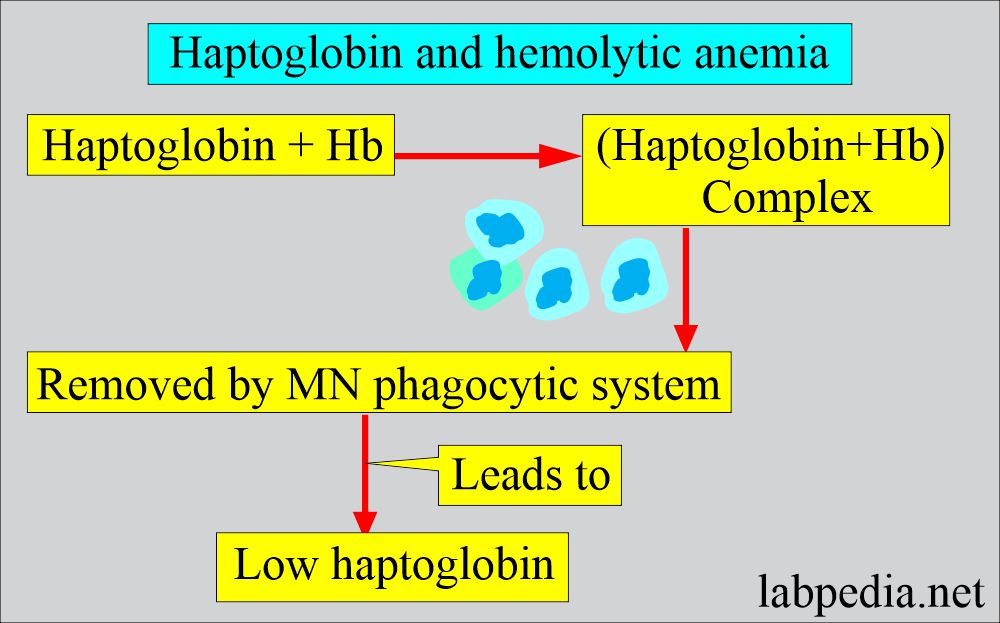
- Decreased plasma haptoglobin.
- There is spherocytosis.
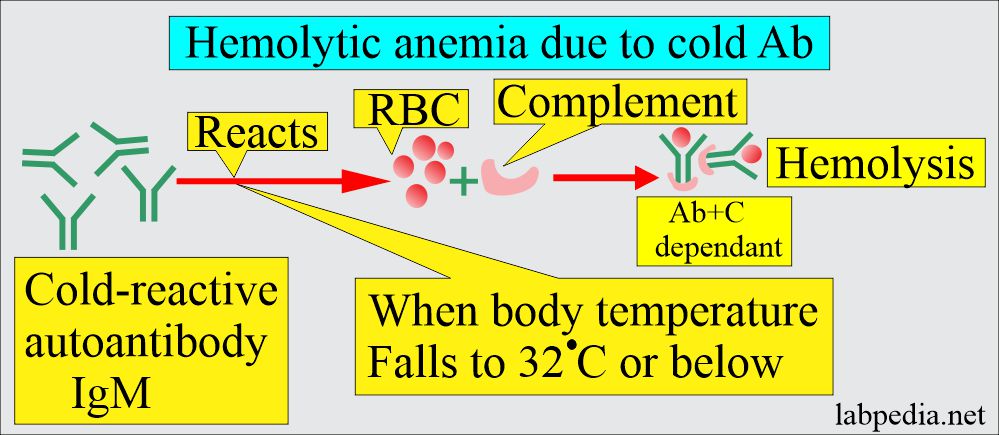
B: Cold antibody Autoimmune hemolytic anemia:
- These antibodies react more strongly at 4 °C.
- This reaction usually takes place below 37 °C.
- The antibody IgM is mostly cold agglutinin reacting at 4 °C in the plasma.
- Mechanism: At low temperatures, these antibodies can attach to RBCs and cause their agglutination in the body’s cold periphery, like hands and feet.
- In addition, there is the activation of the complement, and this will lead to intravascular hemolysis.
- After the Mycoplasma, cytomegalovirus, and Epstein-Barr virus infection, there is an increased synthesis of polyclonal cold agglutinins.
- These cold, cold agglutinins lead to mild to moderate transient hemolysis.
- Chronic cold haemagglutinin disease is usually seen in older adults. This is due to the production of the monoclonal IgM cold agglutinins.
- Coomb’s test is positive but not strongly.
- This may occur from infections like infectious mononucleosis, Mycoplasma pneumoniae, and rarely viral infection.
- This condition may be from lymphoma and paroxysmal cold hemoglobinuria (due to IgG).
- Diagnosis:
- RBCs agglutinate at cold temperatures or room temperature. Sometimes when you keep the sample at a cold temperature, it will show agglutination.
- Direct Coomb’s test is positive with complement.
- Treatment:
- Try to find the underlying cause.
- Keep the body parts warm and avoid cold exposure.
- Treatment with steroids, alkylating agents, and splenectomy does help these patients.
Clinical signs and symptoms of autoimmune hemolytic anemias:
- This may be seen at any age and sex, and anemia is of varying severity.
- There may often be splenomegaly.
- Usually, there are attacks and remissions.
- It may start after some drug therapy like methyldopa.
- It may be associated with other diseases Like SLE and ITP.
- In SLE, the cells are coated with immunoglobulins and complements.
Hemolytic anemia, lab diagnosis:
- The lab findings can be divided into three broad categories:
- Evidence for increased RBCs breakdown:
- Increased serum bilirubin and mainly unconjugated bilirubin (indirect bilirubin).
- Excessive urobilinogen is excreted in the urine (this is due to the breakdown of the bilirubin in the intestine).
- Fecal stercobilinogen is also increased in the stool.
- There is an absence of serum haptoglobin due to saturation with hemoglobin, and the MN phagocytic system removes it.
- Raised level of lactate dehydrogenase (LDH).
- Increased production of RBCs (Erythroid hyperplasia):
- Increased reticulocyte count (reticulocytosis). The normal range is 0.5% to 2%.
- They leave the bone marrow and go to peripheral blood, where they get maturation, which is more than the normal of one day. This will lead to a false increase in the reticulocyte count.
- There is bone marrow erythroid hyperplasia with changed M: E (normal = 2:1 to 12: 1) is decreased to 1:1, or there is a reversal.
- Increased reticulocyte count (reticulocytosis). The normal range is 0.5% to 2%.
- Evidence of damaged RBCs:
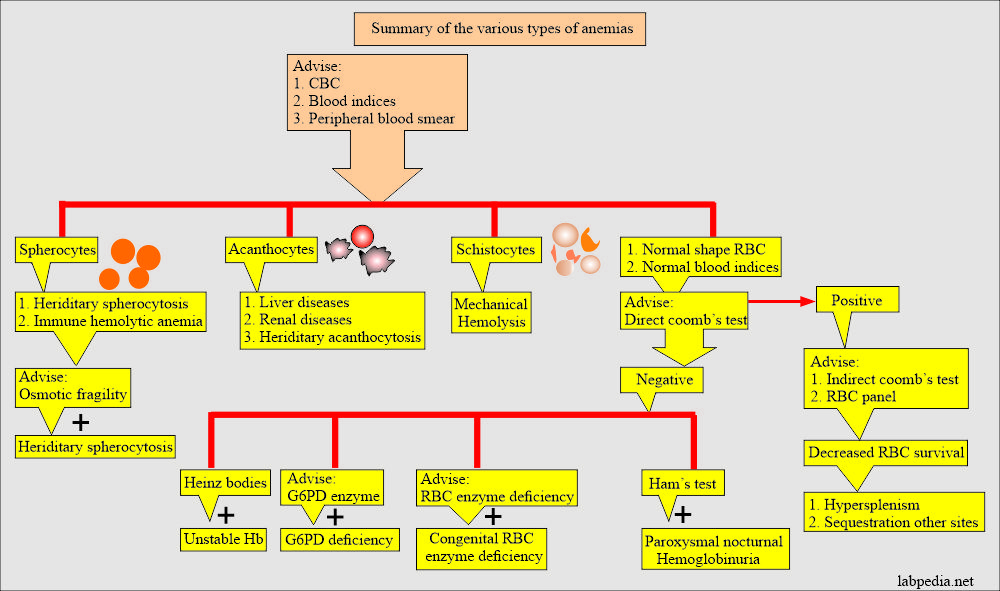
- Morphologically will see elliptocytes, fragmented RBCs, and microspherocytes.
- Osmotic fragility is increased.
- RBC survival is shortened and can be studied by labeling the RBCs with 51Cr. It also can identify the site of destruction.
- Separate the patients into groups:
- Coombs test positive group, e.g., immune hemolytic anemia.
- Coombs negative group, e.g., non-immune hemolytic anemia.
- Hb is reduced and may be mild to moderate, decreased from 6 to 10 G/dL.
- Reticulocytes are increased from 5% to 20 % (Reticulocytosis).
- MCV is normal or slightly increased,
- MCHC is increased.
- Bone marrow shows erythroid hyperplasia.
- Normal myeloid: the erythroid ratio of 2:1 to 12:1 is reduced to 1:1 or much more reduced.
- Coomb’s test direct is negative, which differentiates it from autoimmune hemolytic anemia.
- The peripheral blood smear shows poikilocytosis and polychromatophilia due to reticulocytes.
- There are elliptocytes and fragmented RBCs.
- There are normochromic and normocytic anemia pictures.
- RDW increases due to anisocytosis and poikilocytosis, unlike other normochromic and normocytic anemias.
- Increased reticulocytes lead to increased MCV but not like megaloblastic anemias.
- Serum bilirubin is raised.
- Urine urobilinogen is positive.
- Stercobilinogen is increased
- Serum haptoglobin is absent.
Lab findings in hemolytic anemia:
| Parameters | Hemolytic anemia |
| Hb | Decreased 6 to 10 g/dL |
| Reticulocytes | Increased 5 to 20% |
| MCV | A normal or mild increase |
| MCHC | Increased |
| Bone marrow | Erythroid hyperplasia |
| Coombs test | Direct is positive in acquired AIH. |
| RDW | Increased |
Treatment of autoimmune hemolytic anemia:
- Remove the cause, like drugs (methyldopa).
- The first treatment line is corticosteroids, and prednisolone, starting at 60 mg/day in adults, then tapered down.
- In the case of complement-associated AIH, the response is poor to steroids and even splenectomy.
- Immunosuppression can be tried when other measures fail. It can give Azathioprine, cyclophosphamide, chlorambucil, mycophenolate, and ciclosporin.
- Monoclonal antibodies, anti-CD20, and anti-CD52 have been tried successfully in a few cases.
- Folic acid is given in severe cases.
- Blood transfusion is given in the case of anemia.
- In the case of ITP, immunoglobulin can be given.
Question 1: Where extravascular hemolysis takes place.
Question 2: What are the possible sites of RBC hemolysis.
See more details in Anemia Part 1.