Anemia:- Part 8 – Hemolytic Anemias Classification, Lab Diagnosis
Hemolytic Anemias
What sample is needed for the diagnosis of Hemolytic Anemias?
- Make a fresh blood smear.
- Take blood in EDTA.
- Bone marrow may be advised if needed.
How will you define hemolytic anemias?
- This is a hemolytic state when the RBC’s life in vivo is shortened.
- The presence of anemia depends upon the degree of hemolysis and the response of the bone marrow.
- Hemolytic anemia results from the premature destruction of the peripheral blood RBCs.
How is the normal removal of the RBCs?
- RBC destruction occurs after a mean life span of 120 days when the MN phagocytic system removes these in the extravascular space, especially in the bone marrow, spleen, and liver.
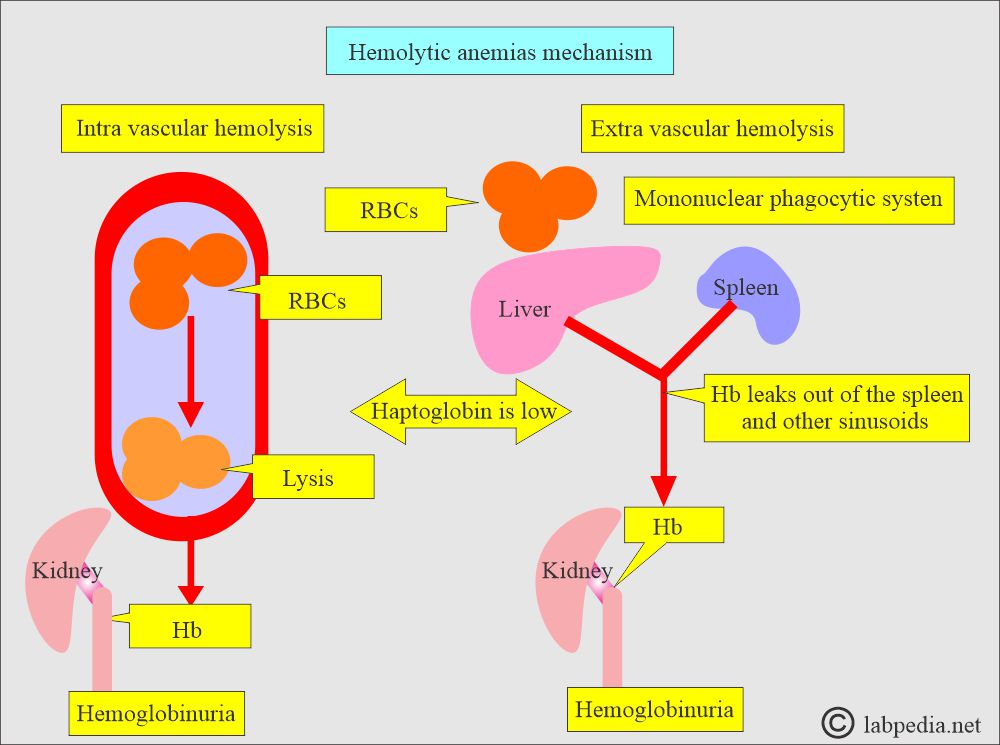
What are the sites of hemolysis?
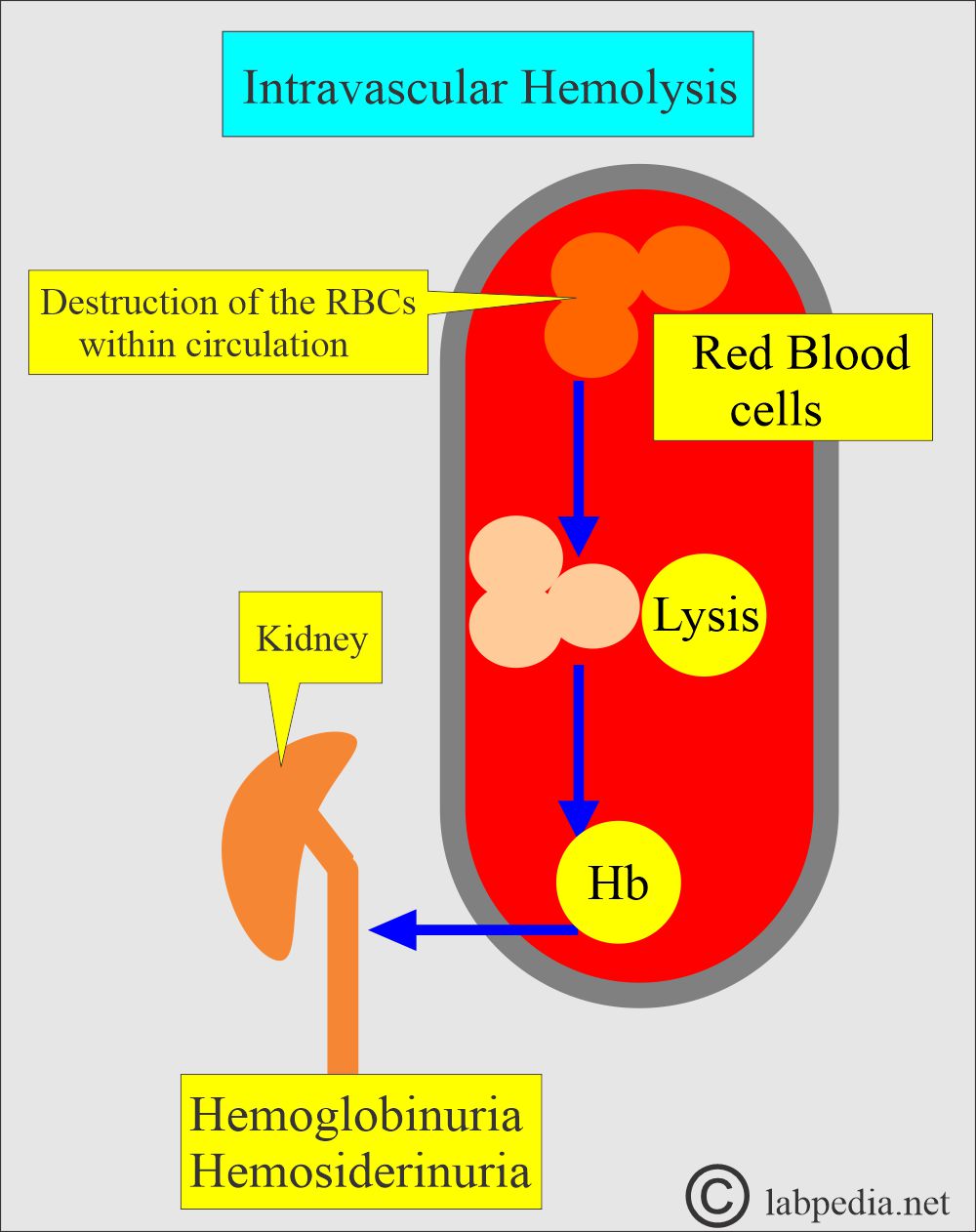
- Intravascular hemolysis occurs when the RBCs are destroyed in the circulation (blood vessels).
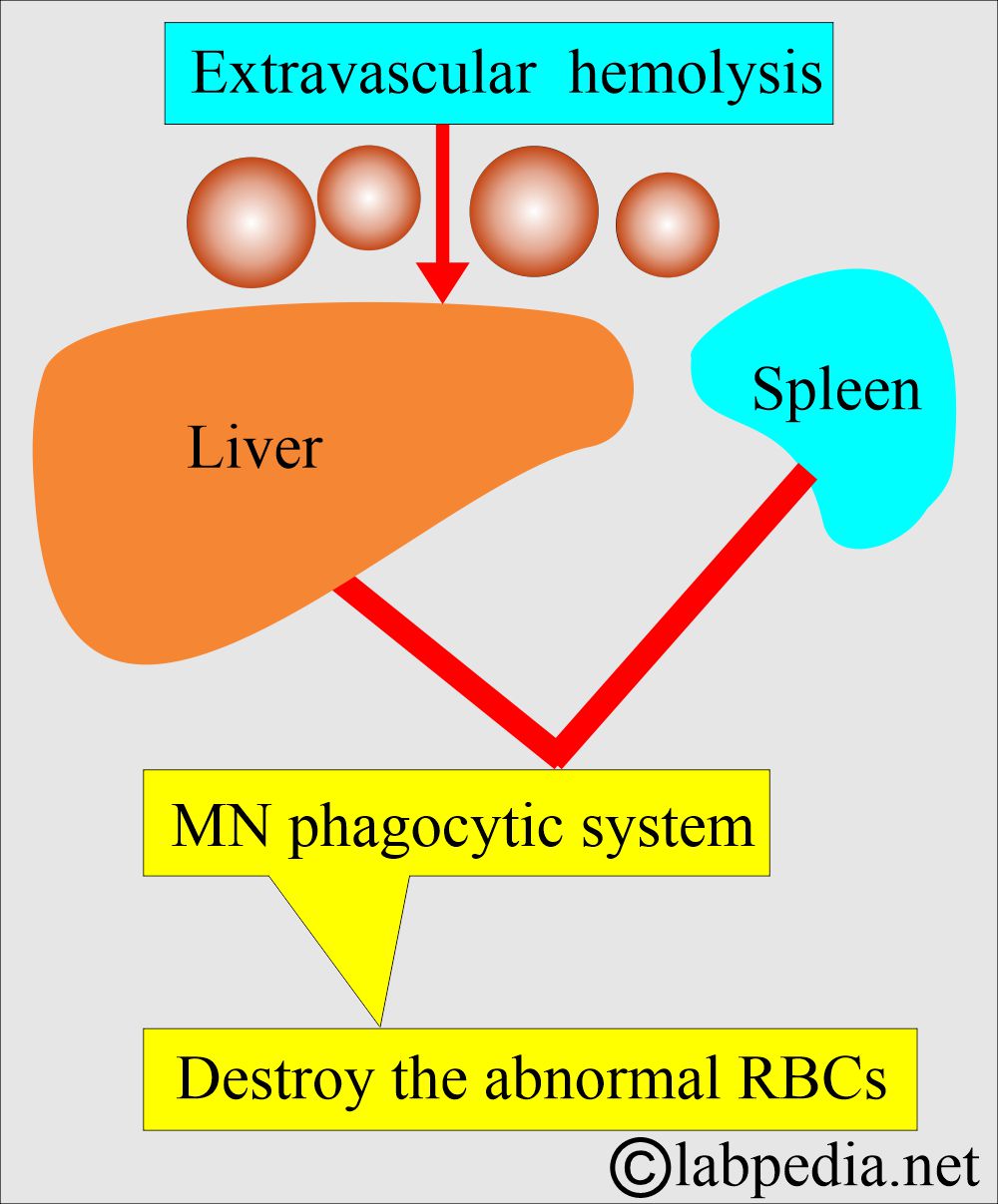
- Extravascular hemolysis occurs when the RBCs are destroyed by the spleen, liver, and bone marrow’s mononuclear phagocytic system.
What is the mechanism of hemolytic anemia?
- The mechanism is defined as increased destruction of the red blood cells.
- Anemia may not appear because of the compensation from the bone marrow.
- When the destruction of RBCs becomes several times greater than this compensatory mechanism, hemolytic anemia will result.
- Hemolytic Anemia may be:
- Intrinsic factors where the RBCs are abnormal.
- Extrinsic factors, which are external factors, can destroy the RBCs.
- The warm antibody reacts in vitro best at 37°C.
- The cold antibody reacts at 4°C to 10°C.
- Hemolytic anemias may be due to:
- Congenital abnormality.
- Acquired defects.
- All these anemias share common defects, which are shortened survival of the RBCs.
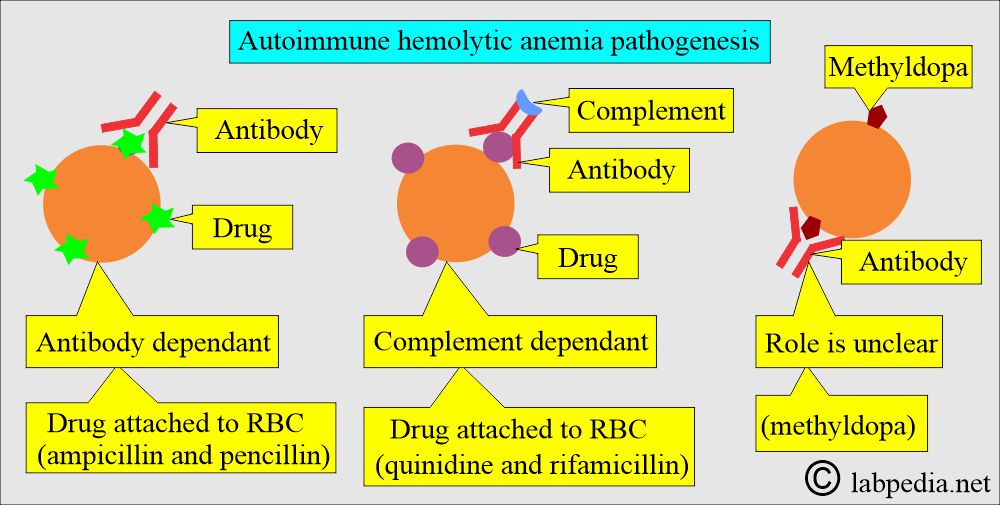
What is the role of drugs and antibodies for hemolytic anemias?
- Combining the drugs with anti-drug antibodies forms the immune complex that is adsorbed onto the RBCs, often activating the complement.
- The antiquinidine antibody is IgM.
- The drugs bind to the RBCs’ membrane, acting as haptens.
- Penicillin is the best example. This type of reaction develops in a few cases, around 3%.
- There may be a nonspecific coating of the RBCs by drugs and various proteins.
- Antibiotics like Cephalothin act on this principle.
- The unknown mechanism by which α-methyldopa leads to hemolytic anemia.
- The antibody against the α-methyldopa is IgG, which usually has Rh group specificity.
What is the mechanism of hemolytic anemia?
- Some conditions produce hemolytic anemia, forming many schistocytes, which may form due to trauma.
- The common examples are:
- Disseminated intravascular coagulation (DIC).
- Thrombotic thrombocytopenic purpura.
- The hemolytic uremic syndrome:
- Cardiac prosthesis syndrome.
- Long-term indwelling catheters.
- Hemolytic anemia is associated with a vascular graft.
- In gastric carcinomas.
- In Clostridium welchii septicemia.
- Paroxysmal nocturnal hemoglobinuria (PNH):
- This is an acquired blood cell membrane defect in which RBCs, WBCs, and platelets demonstrate abnormal sensitivity to the effect of activated serum complement.
- These are manifested by hemolytic anemia, leucopenia, and thrombocytopenia.
- PNH affects young or middle-aged adults, with the usual age range of 10 to 60.
- Abdominal pain occurred in about 10% of the cases, and hemoglobinuria occurred in 50%.
- Anemia is usually of a moderate degree except during the acute episode of crises, when it is severe.
- Hemolytic anemia due to toxins:
- Lead poisoning is the most frequent cause.
- This is usually seen in the ingestion of paint, which is frequent in children.
- Hemolytic anemia due to bacteria:
- This is usually seen in septicemia due to Clostridium welchii.
- This may produce severe hemolytic anemia with the presence of spherocytes.
- Hemolytic anemia due to parasitic infestation:
- The most frequent cause is malaria.
- Bartonella infection may cause hemolytic anemia.
- This is most common in Peru.
What is the outcome of hemolysis?
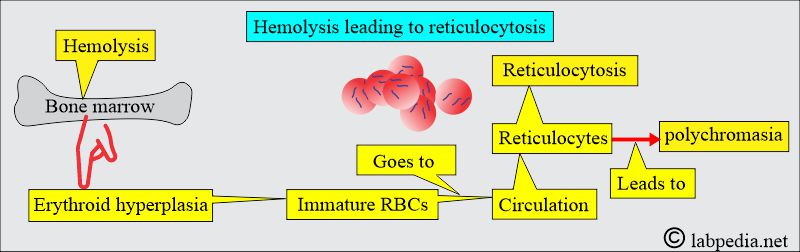
- If the bone marrow hyperplasia compensates for the RBC loss, then no anemia will develop, which is called compensated hemolytic disease.
- The bone marrow becomes hyperplastic, and erythropoiesis increases 6 to 8 times the normal. The volume of active marrow also increases.
- Immature RBCs (reticulocytes) are released prematurely into the circulation and give rise to polychromasia.
How will you classify hemolytic anemia?
- Anemia due to isoagglutinin (isoantibodies).
- The antibodies within blood group systems cause these anemias.
- Anemia due to autoagglutinins (autoimmune hemolytic anemia or acquired hemolytic anemia):
- Antibodies are produced against the self-own body tissue.
- Warm autoantibodies. These are IgG and are usually directed against the Rh-antigen.
- These 50% to 70% are Coombs’ positive autoantibodies.
- Cold autoantibodies. These are IgM antibodies usually directed against the I antigen on the RBC membranes.
- These are 15% to 30% of Coombs’ positive autoantibodies.
- Paroxysmal cold hemoglobinuria (PCH).
- This is a rare syndrome.
- The antibodies of the IgG class bind to and sensitize RBCs at cold temperatures.
- It produces complement-activated RBC hemolysis at warmer temperatures.
- PCH is 2% to 5% of Coombs-positive autoantibodies.
- These are more common in children than in adults.
- Hemoglobinuria is produced after exposure to cold.
- Secondary acquired autoimmune hemolytic anemia.
- This may be a cold or warm variety. This can be divided into 3 main groups.
- Due to leukemia or lymphoma.
- Most often, it is due to chronic lymphocytic leukemia.
- Less frequent lymphocytic lymphoma.
- Rarely Hodgkin’s lymphoma.
- This is due to collagen diseases like SLE (systemic lupus erythematosus).
- Miscellaneous systemic diseases like viral infections, severe liver diseases, ovarian tumors, and carcinomatosis.
- Drug-induced hemolytic anemia.
- Sometimes, drug-induced hemolytic anemia is included in autoimmune hemolytic anemia.
- Antibodies are formed against the drugs, and the action on the RBCs is secondary due to the presence of drugs on the surface of RBCs.
- There are 10% to 20% of Coombs-positive autoantibodies.
How will you classify Hemolytic Anemias based on the Coombs’ test?
| Coomb’s positive | Coomb’s negative |
Antibody-dependent hemolysis:
|
Non-antibody dependent hemolysis: Intrinsic RBC abnormalities:
Extrinsic RBCs mechanism:
|
What is the etiology of RBC hemolysis?
- Hereditary is due to intrinsic red cell defects.
- Acquired are due to environmental changes.
- Hemolytic anemia can also be divided into:
- Anemias are associated with intrinsic/intracorpuscular defects of RBCs.
- The life of the RBCs in patients and even in the recipient is shortened.
- Anemias are associated with extrinsic/extracorpuscular defects of RBCs.
- In these cases, if normal RBCs are transfused, they will be destroyed more rapidly.
- Anemias are associated with intrinsic/intracorpuscular defects of RBCs.
- Intravascular hemolysis:
- It occurs when the RBCs are destroyed in the blood vessels, and hemoglobin is released.
- This is associated with physical trauma, like an artificial heart valve.
- Or this is associated with the complement system, like paroxysmal nocturnal hemoglobinuria.
- Hemoglobin in the blood gives rise to hemoglobinuria.
- What are the causes of intravascular hemolysis?
- Mismatched blood transfusion.
- G6PD deficiency is associated with stress.
- March hemoglobinuria.
- Few autoimmune hemolytic anemias.
- Extravascular hemolysis:
- When the destruction of the RBCs takes place in the mononuclear phagocytic system.
- The MNphagocytic system will trap and disrupt the abnormal cells.
- The spleen is very efficient in this respect and is followed by the liver.
- That is why there is splenomegaly in hemolytic anemia (disease).
- Abnormal hemoglobin:
- The hemoglobin function abnormalities and Heinz body formation cause the RBCs to lose their elasticity. When such RBCs go through the microcirculation, the RBC membrane cannot adjust to the small blood vessels, and they are trapped and destroyed.
What are the causes of Hereditary hemolytic anemia?
- RBC cell membrane defect:
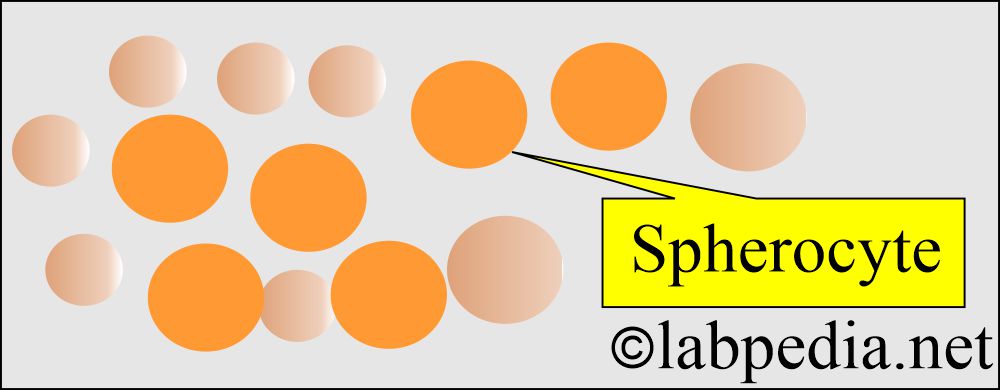
- Abnormality in the cell membrane cytoskeleton leads to spherocytosis and elliptocytosis.
- Abnormal lipid synthesis is like an increase in the membrane lecithin.
- RBC cell enzyme deficiency:
- G-6-PD deficiency.
- Pyruvate kinase deficiency.
- Hexokinase deficiency
- Glutathione synthetase deficiency
- Disorders of hemoglobin synthesis:
- Hemoglobin synthesis defects like Thalassemia.
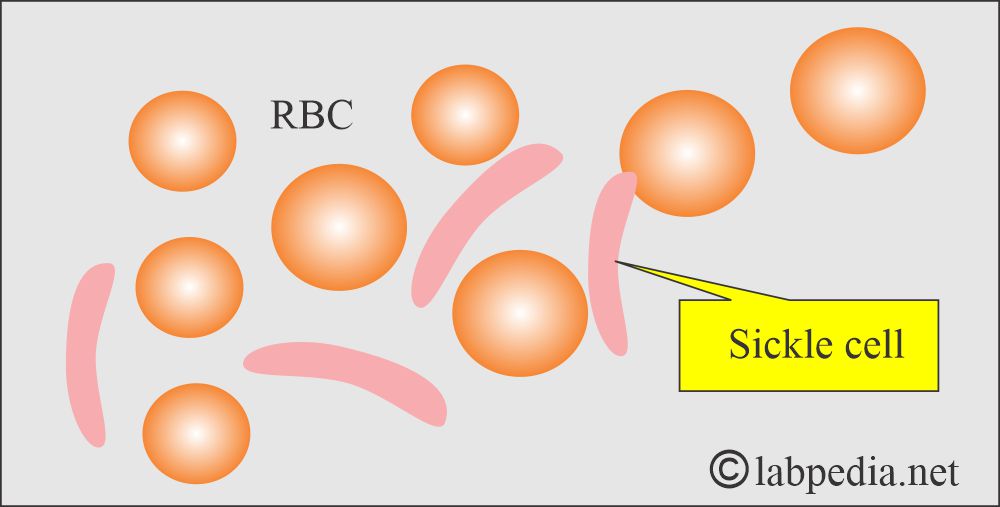
- Abnormal structure of the hemoglobin-like Sickle cell anemia, Hb E.
What are the acquired Causes of hemolytic anemia?
- Antibody-mediated cytotoxic reaction (Type II) like ABO incompatibility.
- Hemolytic autoimmune anemia is seen with cold antibodies and warm antibodies.
- Drug-induced hemolytic anemia is seen in Methyldopa, penicillin,
- Microangiopathic hemolytic anemia is seen due to RBCs’ trauma in the cardiac prosthetic valve and fibrin deposition in the microvasculature.
- Infections: Hemolytic anemia due to toxins like a malarial parasite, septicemia, Clostridium Welchii, pneumococci, staphylococci, and lead poisoning.
- Hemolytic anemia is due to snake venom and spider bites.
- Hemolytic anemia due to lead poisoning and copper toxicity.
- Hemolytic anemia due to extensive burns.
- Hemolytic anemia in splenomegaly.
- Paroxysmal nocturnal hemoglobinuria.
- Anemia due to acquired RBC membrane abnormality.
- Secondary to liver and kidney diseases.
What are the causes of hemolytic anemia?
| Congenital causes of hemolytic anemia (inherited) | Acquired causes of hemolytic anemia |
|
|
What is the other classification of hemolytic anemia?
| Intracorpuscular causes | Extracorpuscular causes |
|
|
|
What is the clinical presentation of hemolytic anemia?
- There is pallor of the mucous membrane, which can be judged from the tongue.
- There is fluctuating jaundice. In some patients, bilirubin will be very high and may need blood transfusion exchange.
- Urine shows no bilirubin, but there is the presence of urobilinogen.
- Gallstones are common in these patients.
- Patients with sickle cell disease may develop ulcers around the ankle.
- Aplastic crises may be seen in these patients.
- Rarely does folate deficiency cause aplastic crises.
Autoimmune hemolytic anemia:
How will you define Autoimmune hemolytic anemia?
- These are caused by the production of the antibody against self-RBC antigens.
- These are characterized by the direct Coombs test (direct antiglobulin test) and divided into:
A: Warm antibody, Autoimmune Hemolytic anemia:
- Reacts more strongly at 37 °C.
- The type of antibody is IgG.
- Coomb’s test is strongly positive.
- Mostly, the cause is idiopathic.
- This is an autoimmune phenomenon, and the best examples are SLE, lymphoma, Hodgkin’s disease, and drugs (methyldopa).
- Signs and symptoms:
- 30% of the cases’ cause is not known.
- These anemias may occur at all ages and in both sexes.
- But more common in middle-aged females.
- There is an attack of relapse and remission with a short period of anemia and jaundice.
- This may progress to the chronic form.
- It is usually splenomegaly.
- Infection or folate deficiency may lead to a fall in hemoglobin.
- Diagnosis:
- There is reticulocytosis.
- Bone marrow shows erythroid hyperplasia.
- Raised serum bilirubin level.
- Increased urinary urobilinogen.
- Decreased plasma haptoglobin.
- There is spherocytosis.
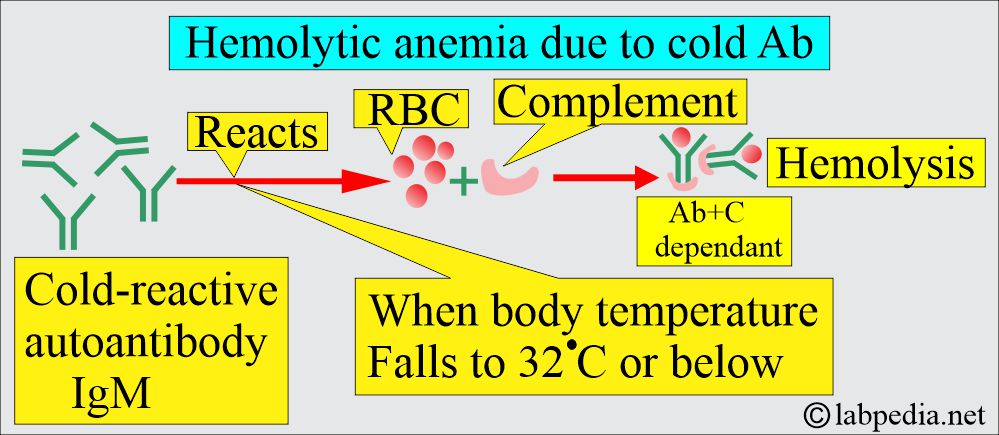
B: Cold antibody, Autoimmune hemolytic anemia:
- These antibodies react more strongly at 4 °C.
- This reaction usually takes place below 37 °C.
- The antibody IgM is mostly a cold agglutinin reacting at 4 °C in the plasma.
- Mechanism of cold antibody hemolytic anemia:
- These antibodies can attach to RBCs at low temperatures and cause agglutination in the body’s cold periphery, like hands and feet.
- In addition, the complement activation leads to intravascular hemolysis.
- After Mycoplasma, cytomegalovirus, and Epstein-Barr virus infection, the synthesis of polyclonal cold agglutinins increases.
- These cold, cold agglutinins lead to mild to moderate transient hemolysis.
- Chronic cold haemagglutinin disease is usually seen in older adults. This is due to the production of the monoclonal IgM cold agglutinins.
- Coomb’s test is positive but not strongly.
- This may occur from infections like infectious mononucleosis, Mycoplasma pneumoniae, and rarely, viral infection.
- This condition may be due to lymphoma and paroxysmal cold hemoglobinuria (due to IgG).
- How will you diagnose a cold-antibody hemolytic anemia?
- RBCs agglutinate at cold temperatures or room temperature. Sometimes when you keep the sample at a cold temperature, it will show agglutination.
- Direct Coombs test is positive with complement.
- How will you treat cold-antibody hemolytic anemia?
- Try to find the underlying cause.
- Keep the body parts warm and avoid cold exposure.
- Treatment with steroids, alkylating agents, and splenectomy does help these patients.
What are the clinical signs and symptoms of autoimmune hemolytic anemias?
- This may be seen at any age and sex, and anemia is of varying severity.
- There may often be splenomegaly.
- Usually, there are attacks and remissions.
- It may start after some drug therapy, such as methyldopa.
- It may be associated with other diseases, like SLE and ITP.
- In SLE, the cells are coated with immunoglobulins and complement.
How will you diagnose Hemolytic anemia?
- The lab findings can be divided into three broad categories:
- Evidence for increased RBC breakdown:
- Increased serum bilirubin and mainly unconjugated bilirubin (indirect bilirubin).
- Excessive urobilinogen is excreted in the urine (this is due to the breakdown of the bilirubin in the intestine).
- Fecal stercobilinogen is also increased in the stool.
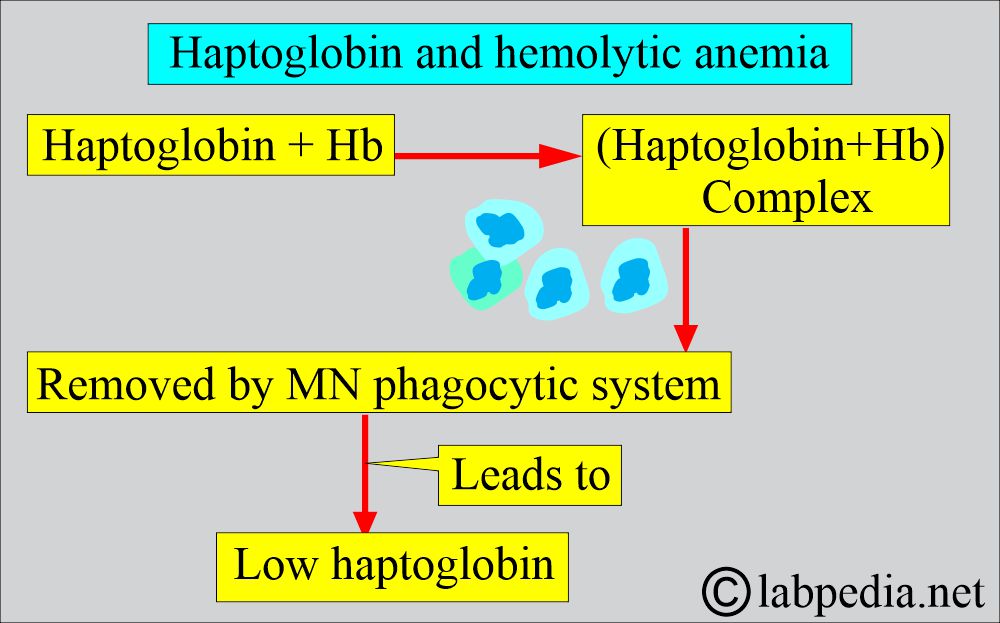
- Serum haptoglobin is absent due to saturation with hemoglobin, and the MN phagocytic system removes it.
- Raised level of lactate dehydrogenase (LDH).
- Increased production of RBCs (Erythroid hyperplasia):
- Increased reticulocyte count (reticulocytosis). The normal range is 0.5% to 2%.
- They leave the bone marrow and go to peripheral blood, where they get maturation, which is more than the normal of one day. This will lead to a false increase in the reticulocyte count.
- There is bone marrow erythroid hyperplasia with changed M: E (normal = 2:1 to 12:1), which is decreased to 1:1, or there is a reversal.
- Evidence of damaged RBCs:
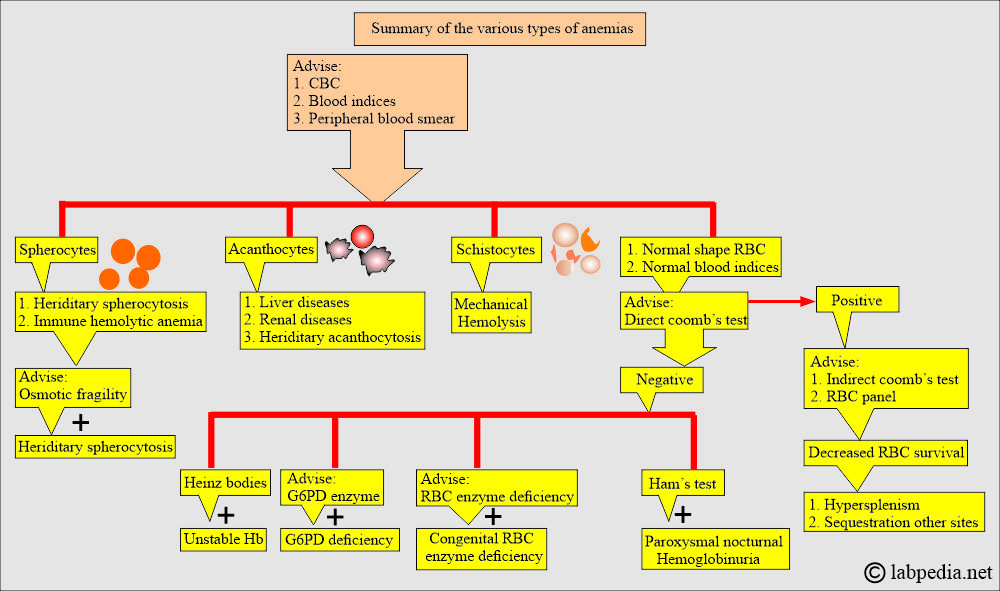
- Morphologically, we will see elliptocytes, fragmented RBCs, and microspherocytes.
- Osmotic fragility is increased.
- RBC survival is shortened and can be studied by labeling the RBCs with 51Cr. It also can identify the site of destruction.
- Separate the patients into groups:
- Coombs test positive group, e.g., immune hemolytic anemia.
- Coombs’ negative group, e.g., non-immune hemolytic anemia.
- Hemoglobin is reduced and may be mild to moderate, decreased from 6 to 10 G/dL.
- Reticulocytes are increased from 5% to 20 % (Reticulocytosis).
- MCV is normal or slightly increased,
- MCHC is increased.
- Bone marrow shows erythroid hyperplasia.
- Normal myeloid: the erythroid ratio of 2:1 to 12:1 is reduced to 1:1 or much more reduced.
- Coomb’s test direct is negative, which differentiates it from autoimmune hemolytic anemia.
- The peripheral blood smear shows poikilocytosis and polychromatophilia due to reticulocytes.
- There are elliptocytes and fragmented RBCs.
- There are normochromic and normocytic anemia pictures.
- RDW increases due to anisocytosis and poikilocytosis, unlike other normochromic and normocytic anemias.
- Increased reticulocytes lead to increased MCV, but not like megaloblastic anemias.
- Serum bilirubin is raised.
- Urine urobilinogen is positive.
- Stercobilinogen is increased
- Serum haptoglobin is absent.
How will you summarize the laboratory findings in hemolytic anemia?
| Parameters | Hemolytic anemia |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
How will you treat autoimmune hemolytic anemia?
- Remove the cause, like drugs (methyldopa).
- The first treatment line is corticosteroids, and prednisolone, starting at 60 mg/day in adults, then tapered down.
- In the case of complement-associated AIH, the response is poor to steroids and even splenectomy.
- Immunosuppression can be tried when other measures fail. It can give Azathioprine, cyclophosphamide, chlorambucil, mycophenolate, and ciclosporin.
- Monoclonal antibodies, anti-CD20 and anti-CD52, have been tried successfully in a few cases.
- Folic acid is given in severe cases.
- Blood transfusion is given in the case of anemia.
- In the case of ITP, immunoglobulin can be given.
Questions and answers:
Question 1: Where extravascular hemolysis takes place.
Question 2: What are the possible sites of RBC hemolysis?
See more details in Anemia Part 1.