Anemia:- Part 4 – Thalassemia, α-thalassemia and β-thalassemia, Workup and Diagnosis
Thalassemia
What sample is needed for Thalassemia?
- Venous blood is needed.
- Prepare a fresh peripheral blood smear.
How will you define thalassemia?
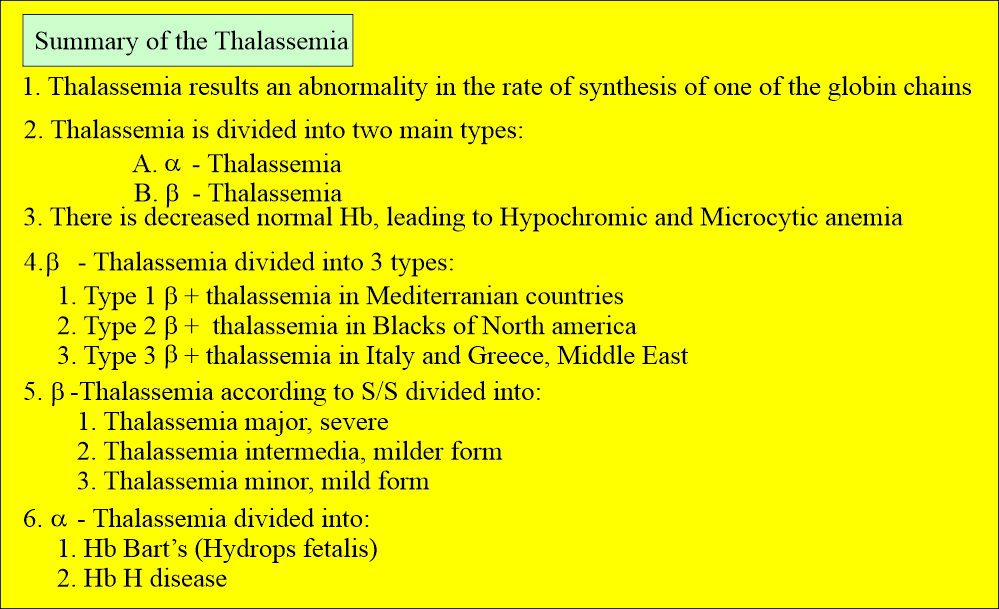
- Thalassemia is an inherited hemoglobinopathy resulting from the decreased production rate of one or more globin chains of hemoglobin. Or
- These are a heterogeneous group of genetic disorders resulting from the decreased synthesis of α or β chains of hemoglobin.
What will be the result of decreased hemoglobin synthesis?
- Decreased hemoglobin in the RBCs.
- Hypochromasia.
- Microcytosis.
- Variable degree of hemolysis.
- Also called Cooley’s anemia.
What is the history of Thalassemia?
- Thalassemia derives from combining the Greek words Thalassa, meaning sea, and Haima, meaning blood.
- This was known as Mediterranean anemia because it is the most common occurrence in the Mediterranean population.
- This is characterized by a decreased production rate of globin chains, which are classified according to the globin involved.
- The consequence is defective globin chain production.
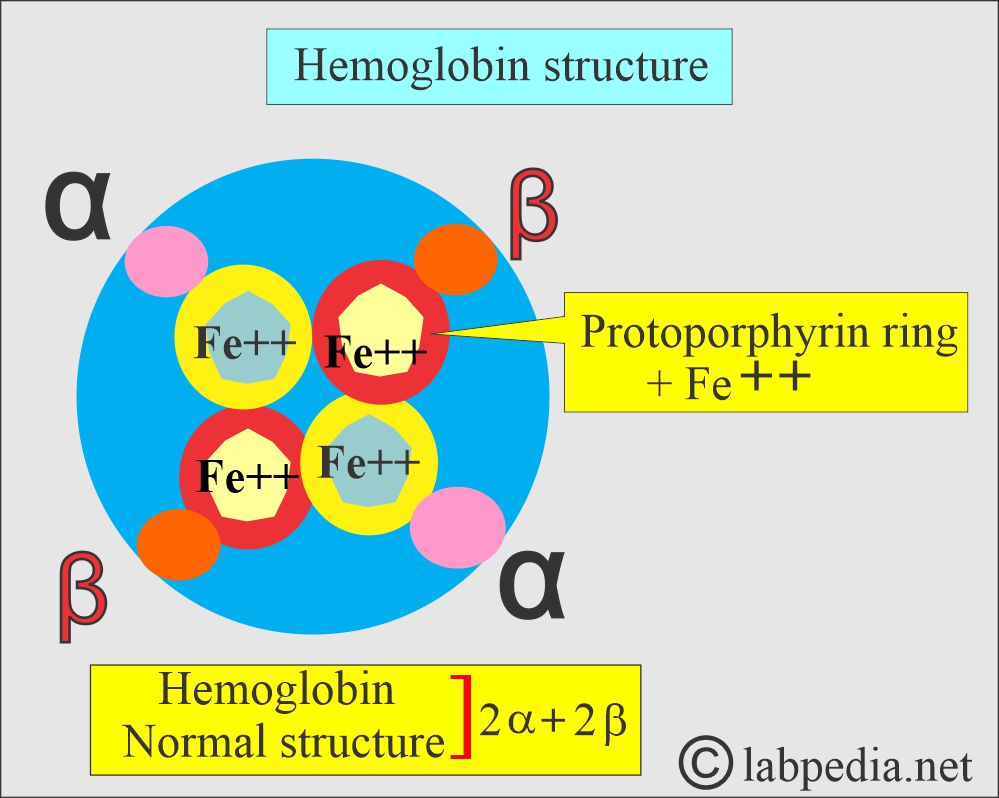
What is the structure of hemoglobin?
- The normal globin, part of the hemoglobin, consists of 2 alpha and 2 beta chains.
What are the various types of hemoglobin and their structures?
| Type of hemoglobin | Genotype of hemoglobin | Hemoglobin presence |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- Each pair is inherited from each parent.
- So one α/β gene is inherited from the father and the other α/β pair from the mother.
- In thalassemia, a gene may involve either α or β chains.
- In the majority of the patients, the β-chain is involved.
- HbA1 has 2 α and 2 β-chains.
- HbA2 has 2 α and 2 δ-chains.
- HbF has 2 α and 2 γ-chains.
- All these hemoglobins, HbA1, HbA2, and HbF, are present in the adult RBCs.
- HbA2 and HbF are present in trace amounts.
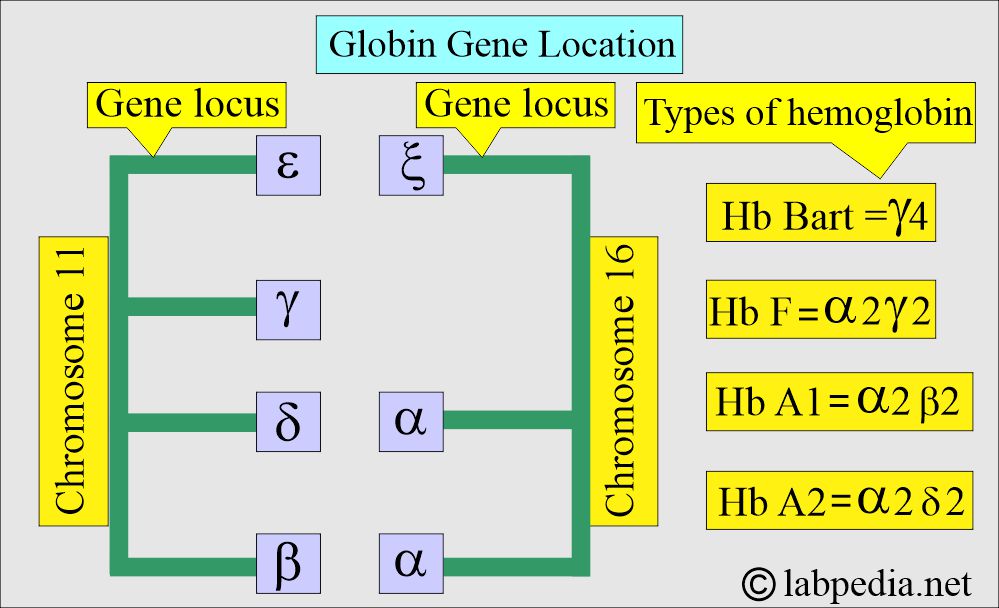
What is the genetic code of Thalassemia?
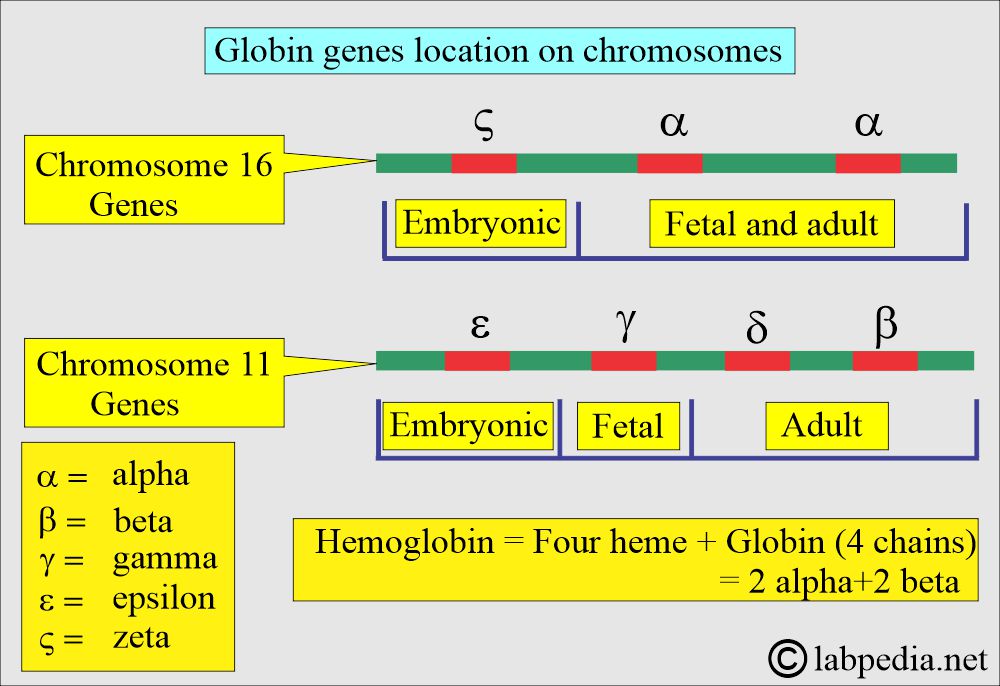
- The genes located on chromosome 11 are γ, δ, ε, and β-chains.
- While on chromosome 16, there are 2 α and ζ loci.
- β-thalassemia:
- Only one of the β-chains is involved in heterozygous conditions, called β-thalassemia minor.
- In homozygous conditions, both β-chains are involved, called β-thalassemia major.
- α-thalassemia:
- α-chain involvement is more complicated.
- Because there are 2 α-gene loci on chromosome 16, while the β-gene is only one locus on chromosome 11.
- In silent carriers of α-thalassemia, only one of the 4 α-genes (1/4) is absent (deleted or abnormal).
- In α-thalassemia minor, 2 of the 4 α-genes (2/4) are affected. There may be a deletion or an abnormality in both gene loci.
- In α-thalassemia-1, which is more common in Asians.
- In α-thalassemia-2, which is more common in Africans and Mediterranean people.
- HbH disease occurs because of the deletion or inactivation of the three gene loci (3/4). Thus, all four globin chains are β-chains.
- Hemoglobin Bart’s disease is more serious when all 4 α-genes (0/4) are deleted or inactivated. There are all 4 γ-globins.
What is the mechanism of Thalassemia?
- Thalassemia syndrome may occur because of the abnormality of the following:
- Coding sequence.
- Transcription.
- Processing or defects in gene translation lead to thalassemia.
How will you classify Thalassemia?
- The older classification classifies thalassemia based on the severity of the disease as follows:
Thalassemia major:
- α-globin genes are absent (0= –/–).
- Hemoglobin (Hb) Bart’s at birth is 75%.
- MCV = 110 to 120 fl.
- MCH is significantly decreased.
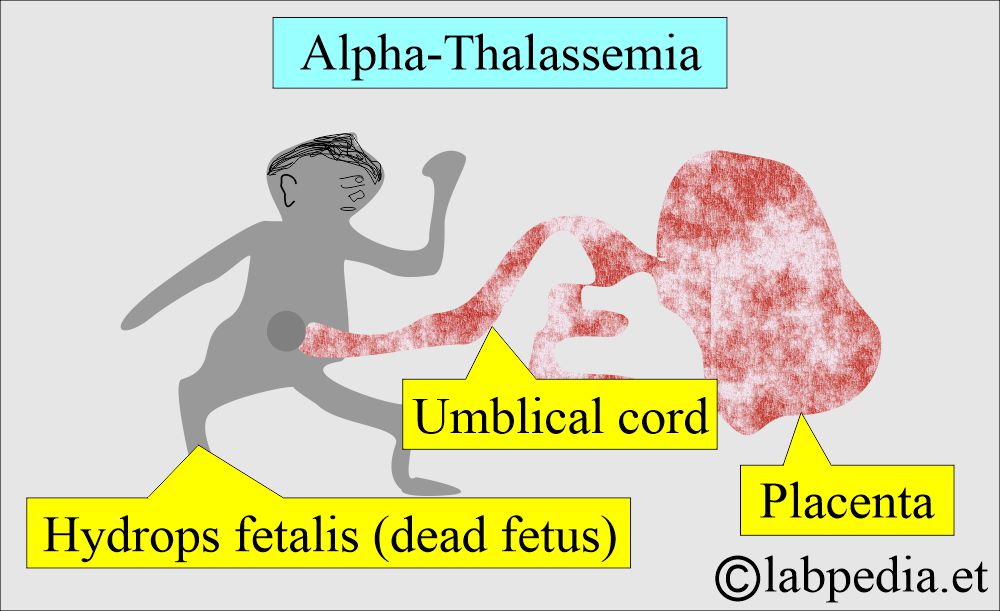
- This is also called hydrops fetalis.
- What are the signs and symptoms of Thalassemia major?
- The complete absence of the α-globin genes in fetal life leads to intrauterine death of the fetus due to severe hypoxemia.
- This is due to Hb Bart’s, which has a high affinity for oxygen and prevents the release of O2 to the tissues.
- At birth, no S/S.
- Infants during 3 to 6 months show pallor, yellow skin, and sclera.
- Infants from 6 to 12 months show severe anemia and bone abnormalities and can’t thrive.
- There are life-threatening complications.
- There is splenomegaly or hepatomegaly.
- These patients will have frequent infections.
- There is a tendency for bleeding, like a nosebleed.
- These patients have a small body, but the large head is a characteristic feature.
- These infants may be mentally retarded.
Thalassemia Intermedia:
- There is some degree of anemia, jaundice, and splenomegaly.
- There are signs of hemosiderosis, such as hemoptysis.
- There is iron deficiency anemia.
Thalassemia minor:
- One globin gene is absent (-α/αα).
- These are the silent carriers.
- There are usually no symptoms.
- There is mild anemia.
- MCV is normal to slightly decreased.
- HbH small amount of 1% to 2% may be present at birth. This will disappear later on.
- Often, these patients are overlooked.
Thalassemia minima:
- It is a mild disease.
- It is a silent carrier of the β-thalassemia trait.
- Anemia is not evident.
- HbA2 = normal or slightly increased. HbF is increased.
- Normal RBC morphology and Hb electrophoresis.
What is the other way to classify Thalassemia?
- It is based on the genetic makeup of the hemoglobin, and it is divided into:
- α-Thalassemia.
- β-Thalassemia
Alpha- thalassemia (α-thalassemia)
How will you define Alpha-Thalassemia?
- α-thalassemia is a group of genetic disorders with defective α-chain synthesis.
- Chromosome 16 carries 2 α genes, and the total number of α-gene is 4.
- Severity depends upon the number of genes affected by the patient, one, two, three, or four.
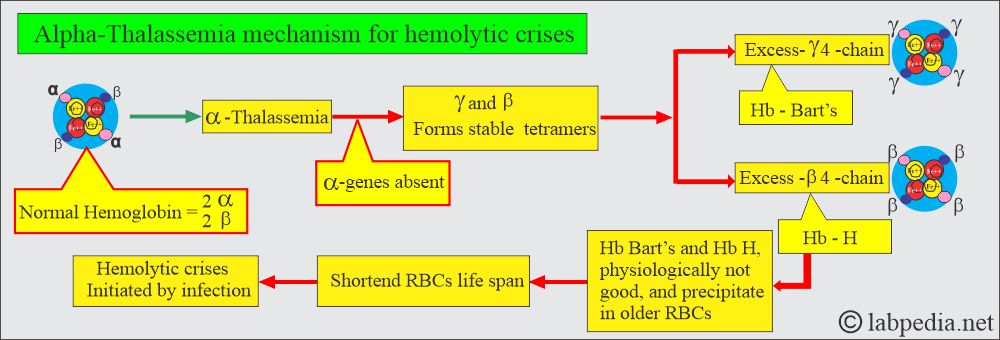
- Decreased synthesis of α-chain will decrease the synthesis of HbA, HbF, and HbA2 because these chains have α-chains; the net result will be an excess of β-chains and γ-chains. These chains may polymerize into tetrameric forms γ4 called Hb Bart’s and β4 called HbH.
- These abnormal Hb Bart’s and HbH are the characteristics of α-thalassemia.
What is the presentation of Alpha-Thalassemia?
- Usually manifested immediately after birth or even in utero because the α-gene is activated early in fetal life.
- α-thalassemia has a wide range of clinical presentations.
- Chromosome 16 carries 2 α genes; the total will be 4 α-genes (each pair from the parents). The severity of the disease will vary depending on the number of genes affected in one patient (one, two, or three genes).
- Another feature of α-thalassemia is that decreased or absent α-gene production results in more than γ-chain during fetal life and at birth, and an excess of β-chain later on.
- This will lead to stable tetramers, γ4 (Hb Bart’s) and β4 (Hb H). Hemoglobin Bart’s and H precipitate in the older RBCs.
- These may lead to hemolytic crises by infection. This abnormal hemoglobin can be detected by electrophoresis.
α-thalassemia minor:
How will you define Alpha-Thalassemia minor?
- These are silent carriers.
- There is decreased production of the α-chain (α+-α / ββ).
- One α-globin gene is affected = -α/αα.
- These are the silent carriers, and there is no marked anemia.
- MCV will be normal, but may decrease slightly.
- Hb H (1% to 2%) is present at birth and disappears later.
α-thalassemia trait:
How will you define the Alpha-Thalassemia trait?
- It has 2 α-globin genes affected = α-/α- or αα/–.
- RBCs show microcytosis and hypochromic anemia.
- MCV is <70fl.
- There is mild anemia.
- Serum electrophoresis showed 5% to 10% Hb H (4 β) at birth, which will disappear later.
α-thalassemia major:
How will you define Alpha-Thalassemia major?
- It is Hb H disease.
- Three α-globin genes are affected = α-/–.
- There is microcytic hypochromic anemia.
- MCV is <70 fl.
- Serum electrophoresis showed predominantly Hb Bart’s, consisting of 4 gamma chains at birth.
- There is a gradual shift from HbH 5% to 30% over the first few months of life.
What are the Alpha-thalassemia characteristic features?
| Clinical features | Genotype structure | Electrophoresis pattern | Peripheral blood smear |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What is the α-thalassemia classification and characteristic features?
| The genotype of α-thalassemia | Severity of anemia | Hb at birth | Hb in adults | α-chain deletion | Clinical outcome |
|
Normal picture |
|
Asymptomatic | ||
|
Hypochromic ± | Hb Bart’s 5% to 10% | Hb A, A2, and F |
|
|
|
Hypochromic +++ | Hb Bart’s 80% | Trace of HbH and Portland |
|
Incompatible with life |
|
Hypochromic ± | Hb Bart’s 1% to 2% | HbA, A2, and F |
|
|
|
Hypochromic ± and inclusions |
Hb Bart’s 1% to 15%
|
HbB 4% to 30% |
|
What are the clinical features of alpha-thalassemia?
- In case of loss of all 4 α-genes, life is incompatible and leads to the fetus’s death (hydrops fetalis).
- Microcytic hypochromic anemia with splenomegaly. This is known as Hb H disease because of the presence of Hb H (β4). Can find this Hb on electrophoresis.
- In fetal life, Hb Bart’s is seen.
- α-Thalassemia trait is caused by the loss of one or two α-genes that are not usually associated with anemia, but MCV and MCH are low.
Beta-thalassemia (β-thalassemia):
Beta-thalassemia major:
How will you define Beta-Thalassemia?
- This is also called Cooley’s anemia and is the homozygous state of β-thalassemia
- It consists of 2 α chains and 2 γ-chains.
- The production of the β-chain is decreased (α2 / β0 β0).
- A globin gene mutation causes partial β-gene or total β-gene chain loss.
- The number of genes affected, partial or complete, will determine the severity of the disease.
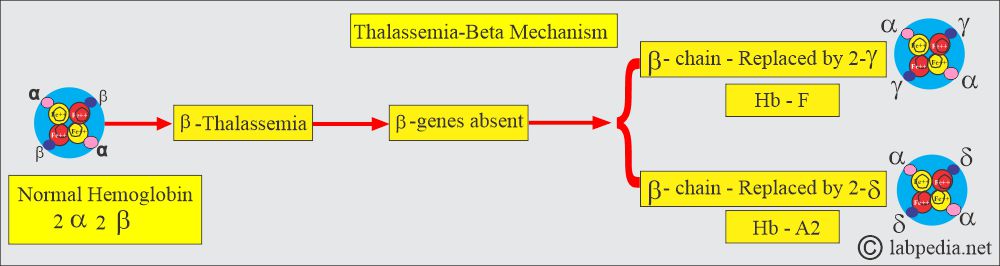
- The production of γ-chains and δ-chains has increased, resulting in increased Hb F and Hb A2 levels.
- The β chain is replaced by the 2-γ chain, which will form Hb F; the other is replaced by δ-chains, which will form Hb A2.
- These are usually homozygous (β0β0):
- β0β0-thalassemia is a more severe variant. No β-chains are synthesized.
- No Hb A found on electrophoresis.
- Only HbF (>90%) and HbA2 (3% to 8%) are found.
- This is also called Cooley’s anemia.
- There is marked microcytosis and hypochromasia.
- MCV is <70 fl, and Hb is 2 to 3 g/dL.
- There is hepatosplenomegaly, bony deformities, and failure to thrive as an infant.
- These patients are dependent upon blood transfusions.
Beta – thalassemia minor:
- Where a single β-gene is affected (β0/β).
- There is mild anemia, Hb 9 to 11 g/dL, or no anemia.
- Normal to increased RBC count.
- RBCs are microcytes, MCV 60 to 70 fl.
- Electrophoresis shows a mild increase in Hb F and Hb A2 (3% to 8%).
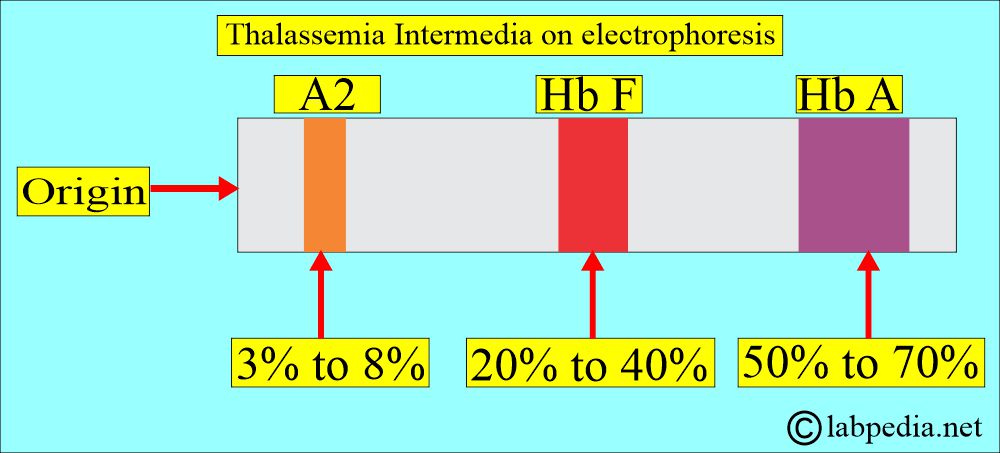
Beta – thalassemia intermedia:
- It is most commonly caused by partial deletion of β0 of both beta genes.
- These are homozygous (β+β+) genes.
- It will give a wide spectrum of the disease with moderate to severe anemia, and Hb will be 6 to 10 g/dL.
- There are growth retardation and bony abnormalities.
- This usually occurs later than the major thalassemia type.
- Electrophoresis shows Hb F 20% to 40% and increased Hb A2, 3% to 8%.
What is another classification of Beta-Thalassemia?
- β0+ shows a complete absence of the production of the beta chains.
- This is found in the Mediterranean, particularly in northern Italy, Greece, Algeria, Saudi Arabia, and Southeast Asia.
- β+-thalassemia is less severe.
- There are three groups of this gene rearrangement.
- 1β+ thalassemia gene produces a smaller amount of the beta-chain, around 10% of normal production.
- This group is found throughout the Mediterranean, the Middle East, the Indian subcontinent, and Southeast Asia.
- The 2β+ thalassemia gene produces more beta-chain, around 50% of the normal population.
- This is found in the blacks of North America and West Africa.
- This is found in the blacks of North America and West Africa.
- The 3β+thalassemia gene produces even more beta chains, leading to milder disease.
- It is found particularly in Italy, Greece, and the Middle East.
- Severe thalassemia is called thalassemia major.
- Severe hypochromic and microcytic anemia develops during the first year of life.
- Hemoglobin is <7 g/dL and consists mostly of HbF and HbA2.
- Homozygous type 2 and 3 beta+ causes a milder form of thalassemia called Thalassemia intermedia.
- The heterozygous beta-thalassemia gene causes a milder form of anemia.
- This also shows mild hypochromasia and microcytosis, called Thalassemia minor.
- The minor group may show a delta-chain abnormality.
- The heterozygous beta-thalassemia gene causes a milder form of anemia.
- Beta-delta Thalassemia (δβ) is another rare form of thalassemia characterized by the combined defect in δ and β chain synthesis.
- This group may have a normal level of Hb A2 and usually a high level of Hb F in the heterozygote and absent Hb A and A2 in the homozygote.
- δβ-thalassemia can be divided into two groups according to the Hb F found:
- If the γ-gene is active, then that group is called GγAγδβ thalassemia.
- Another type that has inactive γ, δ, and β genes is called Gγδβ thalassemia.
What are the findings of Beta-thalassemia syndrome?
| Type of β-thalassemia | Genotype of β-thalassemia | Characteristic features |
|
|
|
|
|
|
What are the clinical features of beta-thalassemia?
- In beta-thalassemia major, there is severe anemia, which appears 3 to 6 months after birth.
- The liver and spleen enlargement is due to increased destruction of the RBCs, intramedullary hemopoiesis, and iron overload.
- Splenomegaly needs more blood and increases RBC destruction and pooling.
- Bone marrow hyperplasia in thalassemia leads to a thalassemic face. Thinning of the bone cortex may lead to bone fractures.
- X-rays may show the bossing of the skull and typically a hair-on-end appearance.
- What are the Lab findings of beta-thalassemia?
- Low Hemoglobin.
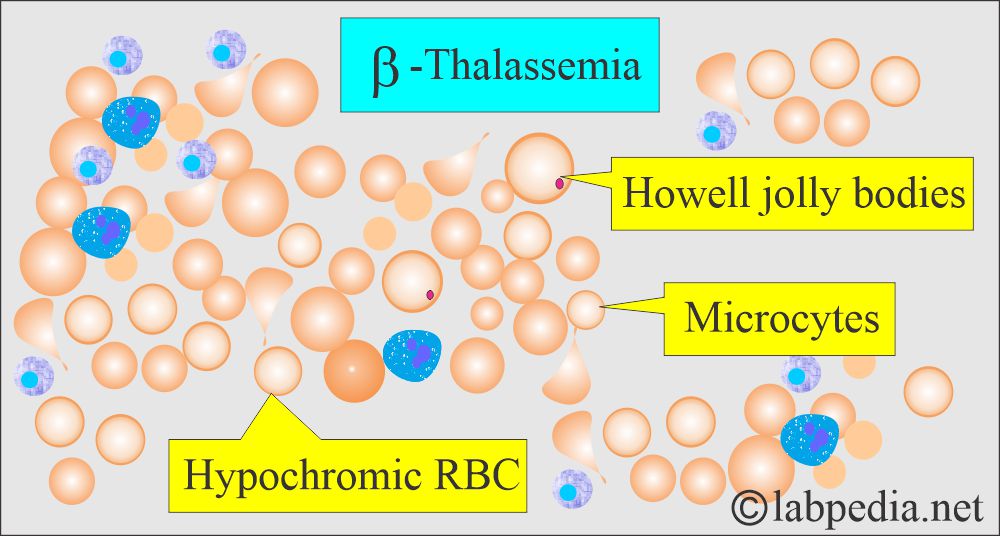
- The peripheral blood smear shows Hypochromic and microcytic anemia.
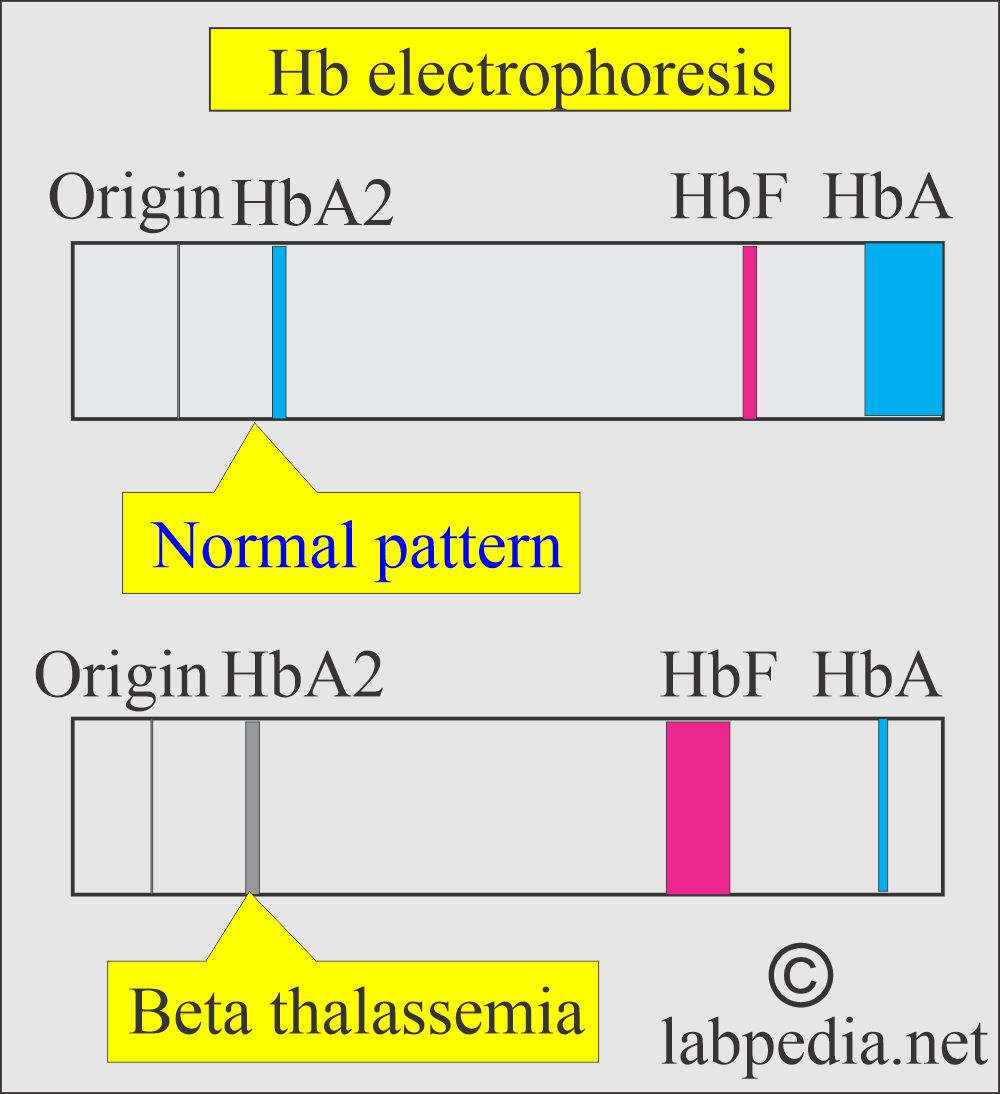
- Hb electrophoresis confirms the diagnosis by the near absence of the decreased level of Hb A.
Hemoglobin on electrophoresis is different in different types of thalassemia.
| Patient | % of the type of Hemoglobin |
|
|
|
|
|
|
|
|
|
|
How will you summarize Thalassemia types?
- α-Thalassemia trait is due to a double gene deletion.
- There are microcytes and hypochromasia.
- α-Thalassemia disease is due to three gene deletions.
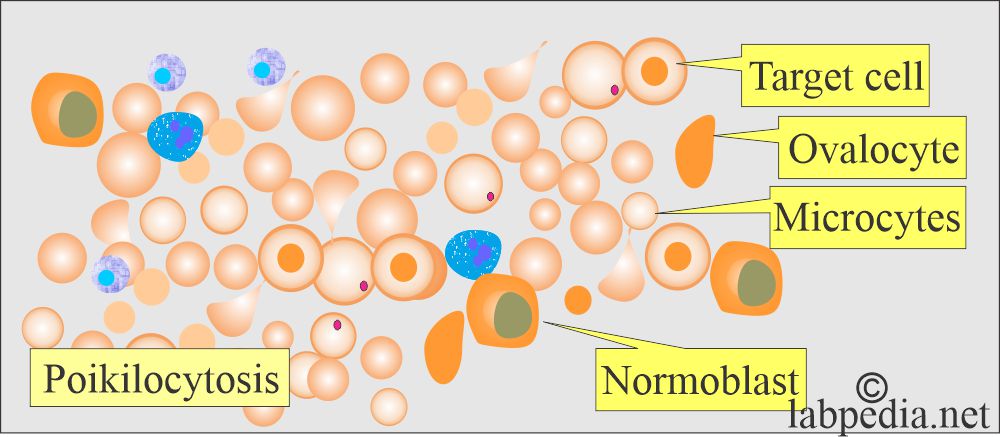
- Target cells, ovalocytes, microcytes, and Hb H are included in the RBCs.
- β-Thalassemia in heterozygotes, and there is a β gene deletion alone or combined with the δ gene.
- There are microcytes, target cells, elliptocytes, and basophilic stippling.
- β-Thalassemia in homozygotes, and there is a β gene deletion alone or in combination with the δ gene.
- It is marked as hypochromasia with polychromatic rims. There are target cells, ovalocytes, basophilic stippling, and HbH crystals.
What is the differential diagnosis of Beta-thalassemia?
| Characteristics | Homozygous β-thalassemia | Heterozygous β-thalassemia |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
How will you treat Thalassemia major?
- These patients survive by blood transfusion. An attempt is made to maintain hemoglobin levels over 10 g/dL.
- It usually requires 2 to 3 units every 4 to 6 weeks.
- Fresh blood, filtered to remove white blood cells, gives the best RBC survival and fewer reactions.
- 500 mL of blood contains 250 mg of iron.
- Regularly give the folic acid 5 mg/day.
- Iron supplements are contraindicated.
- Iron overload is a complication that needs chelating therapy to control iron overload.
- Deferoxamine is the most common drug used for the chelation of iron.
- This can be given 1 to 2 mg with each unit of blood.
- Give 40 mg/kg subcutaneously over 8 to 12 hours, 5 to 7 days weekly.
- This should be started in infants after 10 to 15 units of blood transfusion.
- Excess iron causes skin pigmentation and damages the heart.
- Assessment of the iron status advises:
- Serum ferritin.
- Serum iron.
- % saturation of transferrin.
- Serum non-transferrin-bound iron.
- Assessment of the iron status advises:
- A bone marrow biopsy of reticuloendothelial stores was done using Perl’s stain.
- Liver biopsy for parenchymal and reticuloendothelial stores.
- Assessment of the tissue damage caused by the iron overload:
- For heart damage caused by iron, advice:
- X-ray chest.
- ECG, 24-hour monitoring.
- Echocardiography.
- Radionuclide scans to check left ventricular ejection.
- For liver damage caused by iron, the advice is:
- LFT.
- Liver biopsy.
- CT scan or MRI.
- For endocrine gland damage caused by iron, advice:
- Glucose tolerance test.
- Pituitary gonadotropin release test.
- Growth hormone assay.
- Radiology of the bones.
- Isotope bone density study.
- Functional tests of the thyroid, parathyroid, adrenal, and gonadal glands.
- Vitamin C 200 mg/day. This will help in the excretion of iron produced by deferoxamine.
- Immunization against the Hepatitis B virus.
- Allogenic bone marrow transplantation will give a permanent cure.
- Infections are quite common in these patients and need treatment with antibiotics.
- Treatment for β-thalassemia:
- This is supportive treatment.
- Antibiotics for infections.
- Folic acid supplement.
- Transfusion of packed RBCs to raise the hemoglobin.
- Splenectomy may be advised.
- Bone marrow transplantation may be done.
How to treat Alpha-thalassemia?
- Blood transfusion.
- In utero, a blood transfusion is required in case of hydrops fetalis.
How will you screen and diagnose the thalassemia patient?
- The peripheral blood smear is typical of microcytic and hypochromic anemia.
- In the case of homozygous β-thalassemia and double heterozygous non-α-thalassemia, the peripheral smear shows:
- Severe anisocytosis.
- Poikilocytosis with a bizarre shape.
- There are target cells.
- There are ovalocytes.
- There are numerous nucleated RBCs.
- In heterozygous β-thalassemia, peripheral blood smear shows:
- There are hypochromic microcytic RBCs with moderate anisocytosis and poikilocytosis.
- Basophilic stippling is also seen.
- MCV is the best screening for patients with thalassemia. There is a decreased MCV, with an 85% chance if this is <75 fl (femtoliters).
- RBC count of >5 million/mm3.
- Hemoglobin (Hb) level is <9 g/dL.
- MCHC <30%.
- Reticulocytes are increased in these patients. This is increased in:
- In Hb-H disease may be up to 10%.
- In homozygous β-thalassemia may reach 5%.
- Normal RDW (red cell volume distribution width).
- The only exception to RDW in Thalassemia major is the degree of anisocytosis, leading to an increased level.
- In iron deficiency, the RDW is increased, and the decrease in MCV is less striking.
How will you differentiate various anemias?
| Type of anemia | Serum iron | TIBC | Ferritin level | RDW | Free RBC protoporphyrin | A2 level |
| α-thalassemia | Normal | Normal | Normal | Normal | Normal | Normal |
| β-thalassemia | Normal | Normal | Normal | Normal | Normal | Increased |
| Iron-deficiency anemia | Decreased | Increased | Decreased | Increased | Increased | Normal |
| Chronic diseases anemia | Decreased | Decreased | Increased | Normal | Increased | Normal |
- In heterozygous thalassemia, MCH is <22 pg, MCV <70 fl, and Hb is around 9 to 11 g/dL.
- Cord blood can be used to diagnose thalassemia.
- DNA analysis of the chorionic villus sample at 8 to 10 weeks of pregnancy.
- Or Amniotic fluid cells by amniocentesis at 16 to 18 weeks.
- Serum electrophoresis of the infants.
- Electrophoresis plays an important role in diagnosing thalassemia, and it will detect an increased level of HbA2, HbF, and other abnormal hemoglobins (HbH and Bart’s).
What is the picture of β-thalassemia hemoglobin on Hb-electrophoresis?
| Type of thalassemia | HbA | HbA2 | HbF |
| Normal infants | 95% to 97% | 2% to 3% | 1% to 2% |
| β-thalassemia homozygous type | Nil | 2% to 5% | 95% to 98% |
| β-thalassemia Heterozygous | 90% to 95% | 3.5% to 7% | 2% to 5% |
| β+-thalassemia homozygous or double heterozygous β+/β0 | 5% to 35% | 2% to 5% | 60% to 95% |
| Heterozygousα δβ- thalassemia (Hb Lepore syndrome) | 80% to 92% | 1% to 2.5% | 5% to 20% |
| Hereditary persistent HbF (HPHF) (homozygous) | Nil | Nil | 100% |
| HPHF heterozygous African type | 65% to 85% | 1% to 2.5% | 15% to 35% |
| HPHF heterozygous Greek type | 755 to 85% | 1.5% to 2.5% | 15% to 25% |
How will you summarize thalassemia?
Questions and answers:
Question 1: What is Bart's hemoglobin?
Question 2: What is hemoglobin H (Hb H)?

Good work
Thanks for encouraging remarks.