Ammonia (NH3), Hyperammonia
Ammonia (NH3)
What sample is needed for Ammonia (NH3)?
- Whole blood is needed to estimate ammonia.
- Plasma is prepared in EDTA or heparin other than ammonium heparinate.
- Fasting AM samples are preferred.
- There should be no smoking after 12 midnight.
- Avoid smoking in the vicinity of the test sample place.
- There should be no clinching of the hand.
- Ammonia is a volatile gas that should be transported in ice or tested immediately.
- The specimen may be centrifuged at 4 °C.
- Perform the test within 20 minutes or freeze the plasma immediately.
- A urine 24-hour sample is preferred.
What are the precautions for Ammonia (NH3)?
- Analyze the sample as soon as possible.
- Avoiding hemolyzed samples that increase the ammonia level because the RBCs contain more than three times more than plasma.
- Avoid clenching the fist.
- Avoid exercise before taking the blood sample because it increases the level.
- Don’t smoke at least 8 hours before this test.
- One cigarette smoked one hour before the sample can raise the blood ammonia concentration to 100 to 200 µg/L.
- Smokers need a shower and new clothing.
- The technician should also be a non-smoker.
- The use of the tourniquet may increase the ammonia level.
- Avoid contamination of urine by bacteria or ammonia.
- Glassware should be cleaned and washed with a hypochlorite solution.
- EDTA and heparin are acceptable anticoagulants.
- The arterial blood sample is more reliable than venous blood but is difficult to obtain, so venous blood is taken.
- Drugs that increase the level are:
- Acetazolamide.
- Alcohol.
- Barbiturates.
- Ammonium chloride.
- Narcotics.
- Parenteral nutrition.
- Diuretics.
- Drugs that decrease the level are:
- Broad-spectrum antibiotics (neomycin).
- Levodopa.
- Potassium salt.
- Lactobacillus.
What are the Indications for Ammonia (NH3)?
- To find the progression of liver disease and its response to treatment (Fulminant hepatitis or cirrhosis).
- To diagnose Reye’s syndrome.
- To follow the hepatic encephalopathy.
- To monitor the patient in the case of hyperalimentation, high-calorie I/V nutrition.
- The newborn’s advice is when the infant has irritability, vomiting, lethargy, and develops seizures in the early days of birth.
How will you define ammonia (NH3)?
- Ammonia (NH3) is a colorless gas with a pungent odor. It has one nitrogen and three hydrogen atoms.
- It is highly soluble in water, forming ammonium hydroxide (NH4OH).
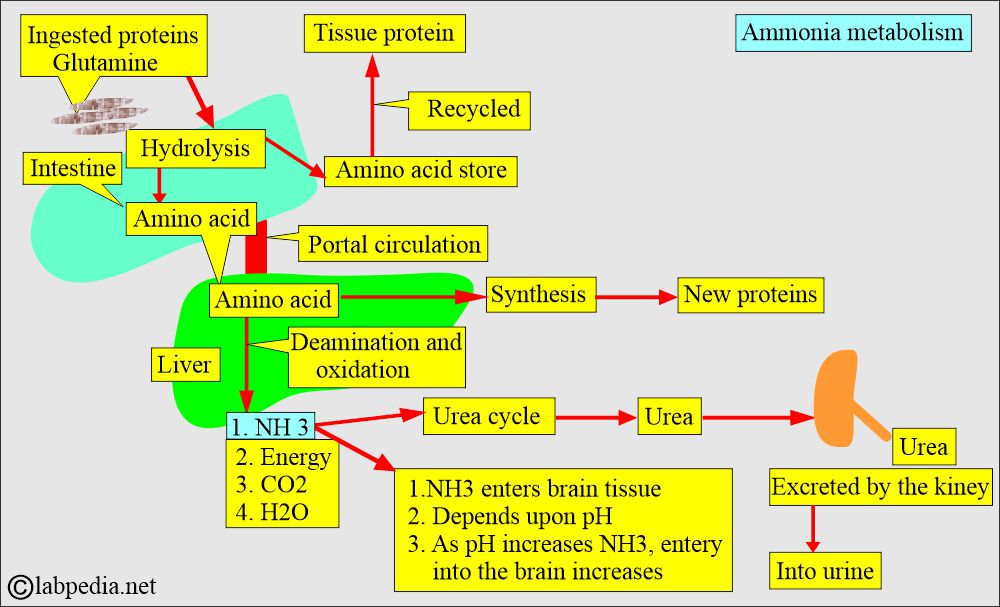
- Ammonia (NH3) is derived mainly from amino acids in the liver via the urea cycle.
- Humans excrete nitrogen from the amino acids and other sources (proteins) as one of the three end products:
- Ammonia (NH3): It is highly toxic, particularly for the brain.
- Urea: Humans are ureotelic (excrete mainly urea as the end product of nitrogenous compounds).
- Uric acid: Birds are uricotelic (excrete mainly uric acid as the end product of nitrogenous compounds).
What are the sources of Ammonia (NH3)?
- Ammonia is the end product of protein metabolism.
- Ammonia in the peripheral blood is present in a very small amount of 10 to 20 µg/dL.
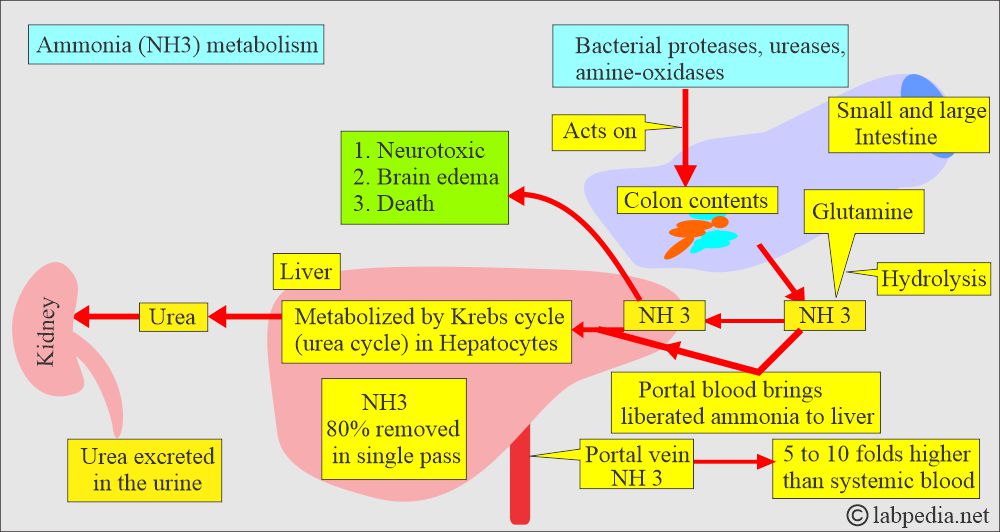
- The main sources of ammonia are skeletal muscles (urea cycle) and the gut, which are derived from the intestinal bacteria that break down proteins.
- Ammonia is produced in the liver, intestine, and kidneys as the end product of protein metabolism.
- Ammonia is a by-product of protein catabolism.
- The major source of NH3 is the gastrointestinal tract.
- In the hepatic portal vein, NH3 concentration is 5 to 10 times higher than the systemic circulation.
- Most ammonia is caused by bacteria acting on proteins in the intestine.
- This intestinal ammonia enters the blood and reaches the liver through the portal vein.
- In portal hypertension, ammonia cannot reach the liver to be catabolized.
What are the Properties of Ammonia (NH3)?
- Ammonia is an inorganic compound of hydrogen and nitrogen with the formula of NH 3.
- NH3 is a colorless, alkaline gas with a pungent smell.
- Ammonia (NH3) is lighter than air and easily liquefied under pressure.
- Ammonia is an irritating gas to the skin, eyes, throat, nose, and lungs.
- Ammonia is the most abundant nitrogen-containing compound in the atmosphere.
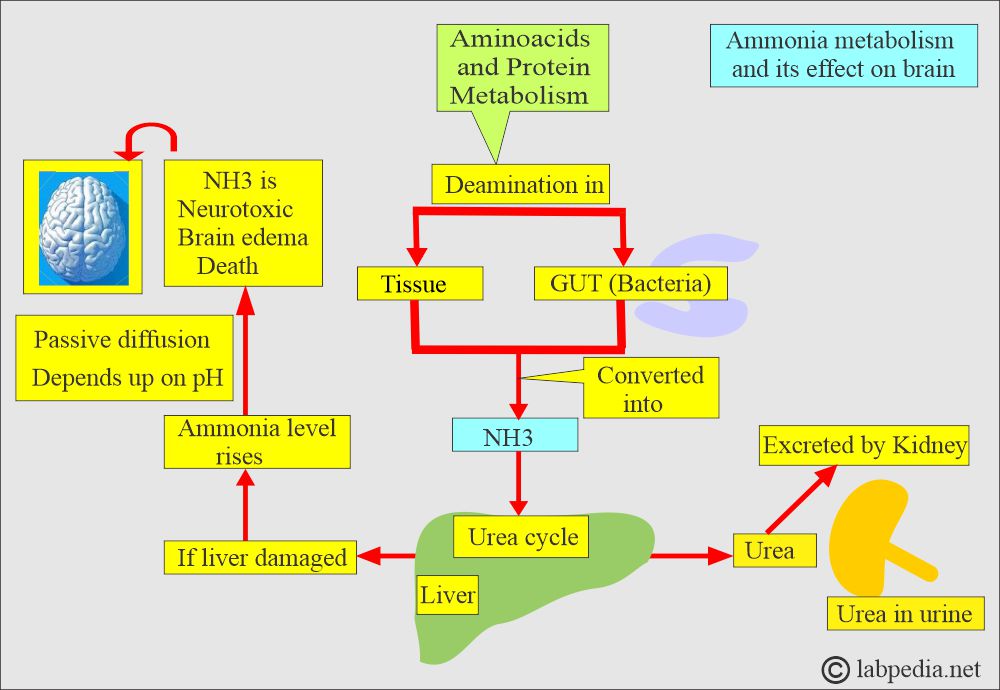
- Ammonia is neurotoxic; it causes brain edema, which may lead to death.
- The liver is the major site for detoxifying ammonia (NH3).
What are the toxic effects of Ammonia (NH3)?
- The liver converts ammonia into urea, which is then excreted by the kidneys.
- If the liver is damaged, then its level increases in the blood.
- It helps diagnose hepatic encephalopathy, and serial estimation may be done to follow the disease.
- Accumulation of ammonia is toxic to the central nervous system.
- The entry of NH3 into nervous tissue depends upon the pH. As the pH increases, the rate of entry of the NH3 into the nervous tissue increases.
- Ammonia (NH 3) crosses the blood-brain barrier more easily than ammonium (NH 4) ions.
What are the uses of ammonia (NH3) gas?
- It is used as fertilizer in the form of ammonium nitrate and urea.
- It is used as a cleaning agent and a household cleaner.
- It acts as a refrigerant gas.
- It is used in the manufacturing of plastics, explosives, and dyes.
What are the signs and symptoms of hyperammonemia (Ammonia, NH3)?
- Hyperammonemia exerts toxic effects on the central nervous system.
- Hyperammonemia Causes may be:
- Inherited. The urea cycle enzyme is deficient and common in infants.
- Acquired. The causes are liver diseases and renal failure.
- There is fatigue.
- There is a loss of appetite.
- Nausea and vomiting.
- There is a loss of strength.
- Ultimately, the patient will become confused.
- The patient may have pain in the abdomen or back.
- Precipitating causes of encephalopathy are:
- Dietary protein.
- Constipation.
- Drugs.
- Infection.
- Electrolytes and acid-base imbalance.
What are the normal levels of Ammonia (NH3)?
Source 2
- Adult = 10 to 80 µg /dL
- Child = 40 to 80 µg /dL
- Newborn = 90 to 150 µg /dL
Another reference
- Normal range = 19 to 60 µg /dL
- Urine = 140 to 1500 µg /dL
Another source
- 19 to 60 µg NH3 /dL (by Du Pont automated clinical analyzer)
- By Ektachem:
- 0 to 10 days = 170 to 341 µg NH3 /dL
- 10 days to 2 years = 68 to 136 µg NH3 /dL
- > 2 years = 19 to 60 µg NH3 /dL
Another source
- Adult = 15 to 56 µg /dL (9 to 33 µmol/L)
- Birth to 10 days = 109 to 182 µg /dL (64 to 107 µmol/L).
- 10 days to 2 years = 95 to 157 µg /dL (56 to 92 µmol/L)
- Children = 36 to 85 µg /dL (21 to 50 µmol/L).
What is the critical value of ammonia (NH3)?
- Ammonia low value = None
- Ammonia high value = >40 µmol//L
What are the causes of raised Ammonia (NH3) levels?
- Raised level of ammonia has toxic effects on the nervous system.
- Hyperammonemia may be due to the lack of the urea cycle enzyme in infants.
- Genetic metabolic disorder of the urea cycle.
- The acquired causes of hyperammonemia are:
- Hepatic coma.
- Reye’s syndrome.
- Hemorrhages like GIT (Gastrointestinal) bleeding.
- Gastrointestinal obstruction with mild liver disease.
- Severe congestive heart failure.
- With congestive hepatomegaly.
- Hemolytic diseases of the newborn (HDN).
- Erythroblastosis fetalis.
- Renal diseases.
- Asparagine toxication.
- Portal hypertension.
- Diuretics and antibiotics may increase the ammonia level.
- Alcohol abuse.
- High temperature (Hyperthermia).
- In the case of hypokalemia (low potassium level).
- Metabolic alkalosis.
- Congenital metabolic disorder of the urea cycle.
- Drugs that increase the level are:
- Alcohol.
- Barbiturates.
- Ammonium chloride.
- Acetazolamide.
What are the causes of decreased Ammonia (NH3) levels?
- Essential or malignant hypertension.
- Drugs that decrease the level are:
- Levodopa.
- Broad-spectrum antibiotic (neomycin).
- Potassium salt.
- Lactobacillus.
- Hyperornithinemia.
Questions and answers:
Question 1: What is the critical value of ammonia (NH3)?
Question 2: What is the role of bacteria in the ammonia formation?
Question 3: Is there any role of pH on ammonia (NH3) toxicity?