Acute Leukemias and their Diagnosis
Acute Leukemias
What sample is needed for Acute Leukemias?
- Blood in EDTA and a fresh blood smear are needed.
- Bone marrow aspirate and biopsy are required.
How will you define acute leukemias?
- Leukemias are characterized by the uncontrolled (malignant) proliferation of the blood and bone marrow cells.
- In the case of a lymphocytic variety, the lymph nodes are involved.
- This is a malignant neoplasm of hematopoietic cells, particularly stem cells.
- There is diffuse infiltration of the bone marrow by these neoplastic cells.
- Leukemias is a malignant hemopoietic tissue disease characterized by replacing normal bone marrow elements with abnormal neoplastic blood cells.
- These cells enter the blood and may infiltrate the liver, spleen, lymph nodes, and other organs.
How will you classify the Acute Leukemias?
- Based on the morphology, the leukemias are divided into:
- Acute lymphocytic leukemia (ALL) involves the lymphocytes and their precursors.
- Acute myelocytic leukemia (AML) involves the granulocytes and their precursors.
- Acute monocytic leukemia involves the monocytes and their precursors.
- Acute plasmacytic leukemia involves the plasma cells and their precursors.
- Acute erythroblastic leukemia involves red blood cell precursors.
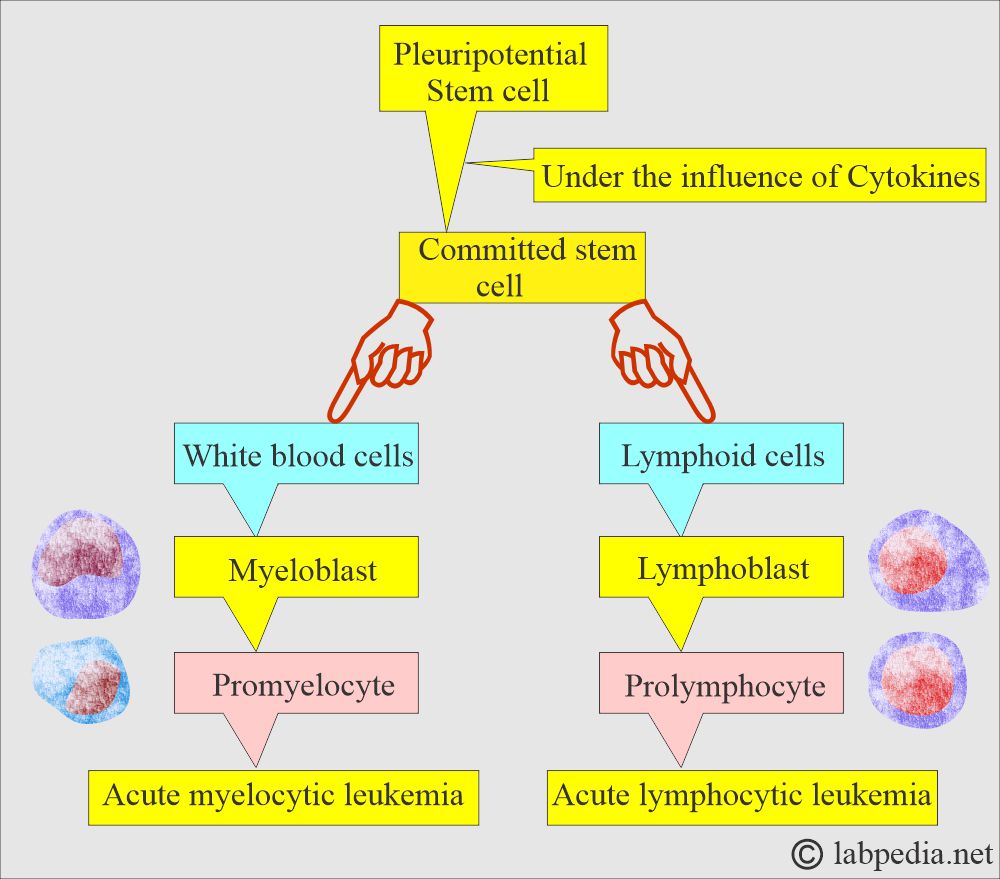
- Acute leukemia develops from the stem cells and transforms into myelocytic and lymphocytic varieties.
How will you discuss the pathophysiology of Acute Leukemias?
- Malignant transformation occurs at the level of pluripotential cells (stem cells), and these cells change into committed stem cells, which is called stem cell disorders.
- Abnormal proliferation, monoclonal expansion, and decreased apoptosis of these cells lead to replacing normal bone marrow cells with malignant or immature cells.
What are the criteria for Acute Leukemias?
- Acute leukemias are characterized by abnormal differentiation and proliferation of malignantly transformed hematopoietic blast cells.
- These cells proliferate in the bone marrow and lead to the suppression of the normal cells.
- These cells have little or no maturation and may see mainly blast cells.
- Acute leukemias are diagnosed based on the percentage of blast cells in the bone marrow.
- 30% or more blast cells are needed to classify the disorder as acute leukemia.
- These leukemias are associated with varying degrees of :
- Anemia.
- Neutropenia.
- Thrombocytopenia.
- Infiltration of the tissues.
- The most common infiltration sites are lymph nodes, liver, spleen, CNS, and skin.
What is the epidemiology of acute leukemias?
- ALL and AML can occur at any age. But the incidence increases with increasing age.
- ALL predominate in children, while AML accounts for most of the cases in adults.
- AML’s median age is around 60 years.
- The peak age for ALL is between 3 to 5 years. These are morphologically different than adult cases.
- Acute leukemias are more common in whites than blacks.
- Acute leukemias are more common in the Jews than in the non-Jews.
- Acute leukemias are more common in men than in women.
What are the etiological factors for acute leukemias (Risk factors)?
- The cause of acute leukemias is unknown; this may be hereditary or environmental factors.
- The pathogenesis is very complex, and it may involve an interaction between the host’s susceptibility and chromosomal damage secondary to physical or chemical agents.
- Chromosomal abnormality:
- There are chromosomal abnormalities; examples are given below:
- Deletion:
- 5q – = Myelodysplasia, preleukemia.
- Monosomy 7.
- Inversion:
- inv 16 = AML.
- Translocation:
- t18:14 = Burkitt’s lymphoma.
- t8:21 = AML.
- t4:11 = ALL.
- t9:22 = CML
- Oncogenes:
- C-abl, C-sis = CML.
- C-myc = Burkitt’s lymphoma (L3).
- Viruses:
- There are viruses known to give rise to leukemia in nonhuman species.
- The human role of viruses has been studied for a long time, but no conclusive data is available.
- Epstein-Barr virus is seen in the majority of Burkitt’s lymphoma.
- The Human T-leukemia virus (HTLV-1) is associated with acute leukemia.
- Inherited factors:
- The following examples favor the role of genetic abnormalities:
- Increased incidence of acute leukemia in families.
- Increased incidence of acute leukemia in monozygotic twins.
- If one twin has leukemia, the other is also at risk for leukemia, often before eight years of age.
- Leukemia starts in the next twin within a year of the first diagnosis.
- Increased incidence of acute leukemias in members of a certain family with high susceptibility, the high increased frequency of leukemia in monozygotic twins, and an association of acute leukemias with genetic disorders all these factors have established that hereditary abnormalities play a role in the development of acute leukemia.
- There is an increased incidence of AML and ALL in Down’s syndrome, where there is trisomy 21.
- 5% to 10% of childhood AML occurs in Down’s syndrome.
- There is an increased incidence of Fanconi’s syndrome and Bloom’s syndrome.
- Both conditions are hereditary, and there is an increased chromosomal breakage.
- Ataxia-telangiectasia and congenital agammaglobulinemia have chromosomal abnormalities that are associated with acute leukemia.
- The following examples favor the role of genetic abnormalities:
- Acquired factors:
- Drugs and Chemicals:
- Alkylating drugs like nitrogen mustard, chlorambucil, melphalan, cyclophosphamide, and procarbazine.
- Benzene compounds and pesticides.
- Exposure to melphalan or nitrogen mustard is associated with acute AML after 5 to 7 years of exposure.
- The patient will have pancytopenia, myelodysplasia, and cytogenetic abnormalities (a total loss or partial loss of chromosomes 5 and 7).
- Hodgkin’s lymphoma, Multiple myeloma, and ovarian cancer treatment complications of AML are well-known examples.
- Benzene complications of acute leukemia are well-known.
- Radiation: The leukemogenic potential of ionizing radiation has been recognized for a long time.
- Mostly, myeloid leukemia takes its origins from radiation.
- Fetal exposure to radiation increases the risk of childhood leukemia.
- Drugs and Chemicals:
| Type of cells | The number of cases | FAB subtype |
|
|
|
|
|
|
|
|
|
What are the signs and symptoms of Acute Leukemias?
- Appears suddenly.
- Suspect leukemia in a patient who has pallor and purpura.
- Patients may have nonspecific symptoms like breathlessness, malaise, and fever.
- The patient may have weakness and fatigue.
- The patient may have a low-grade fever.
- There may be bruising and mild bleeding from the gums.
- There is bone pain due to the expansion of the marrow.
- Children may have bone pain.
- Some patients are severely sick at the time of presentation. These patients may have sepsis and hemorrhage.
What are the clinical features of acute leukemias?
| Pathology | Signs and symptoms |
| Bone marrow failure leads to: | |
|
This will cause:
|
|
This will lead to:
|
|
This will lead to:
|
| What are the features due to metastasis or infiltration of the other organs? | |
|
|
|
|
|
|
|
|
|
|
|
|
How will you diagnose Acute Leukemia?
- These are more common in the younger age group.
- These are more frequent in children.
- Mostly seen before the age of 20 years.
- Onset is an abrupt and fatal outcome.
- There is a fever.
- There is a rapid development of anemia.
- There is normochromic and normocytic anemia.
- May see nucleated RBCs.
- There is thrombocytopenia.
- May see petechiae and purpura in the skin and mucous membranes.
- Leukocyte count is variable.
- The leucocyte count is mostly less than 100,000/cmm.
- Diagnosis depends upon the microscopic examination of the blood and bone marrow.
- Immunohistochemical staining.
- Immunophenotyping by flow cytometry to distinguish T-cell, B-cell, and non-T, non-B cells type of ALL. This is very important because of the response of all these three types of leukemias.
- Cytogenetic studies can also be advised.
- Chromosomal abnormalities are seen in >50% of the cases
How will you differentiate between acute and chronic leukemias?
| Clinical features | Acute leukemia | Chronic leukemia |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
How will you treat acute leukemias?
- The main aim of the therapy is to restore bone marrow function and normality.
- Complete remission when the patient fully recovered and showed in the peripheral blood smear and bone marrow, <5% of the blast cells.
- Induction therapy reduces the total body leukemia cell population from 1012 cells to below <109.
What are the complications of chemotherapy?
- Cytotoxic drugs lead to:
- Tumor lysis syndrome and urate nephropathy.
- Hyperuricemia responds rapidly to rasburicase.
- Allopurinol, I/V therapy, and urine alkalinization reduce the chances of uric acid precipitation in the renal tubules.
- Electrolyte imbalance like hyperkalemia, hypocalcemia, and hyperphosphatemia.
- There may be gastrointestinal injury, diarrhea, and mucositis.
- There may be thrombocytopenia, bleeding, and neutropenic infection.
- Blood products are given to maintain the platelet count >10,000 µL in non-bleeding patients, and hematocrit to keep >25%.
- Antibiotics are given if the body temperature is >38.5 °C.
- In neutropenic patients, when TLC is <500 /µL, antifungal therapy is added to these febrile patients.
Acute Myeloid Leukemia (AML)
What is the pathogenesis of Acute myeloid leukemia?
- There are primary AMLs that appear de novo.
- Secondary AML develops from:
- The myelodysplasia.
- Other hematological diseases, such as myeloproliferative disorders.
- Or following previous treatment with chemotherapy.
- Age: This is seen during the first month of life and in the latter age group.
- 10% to 15% of cases are found in children.
- Exposure to high-energy radiation in early childhood increases the risk of developing T-cell acute lymphoblastic leukemia.
- Sex: More common in males than women.
- Male: female ratio is 3:2.
What are the signs and symptoms of Acute myeloid leukemia?
- Mostly, these patients present with a varying degree of bone marrow failure.
- So, clinical symptoms and signs are due to anemia, leukopenia, thrombocytopenia, and infections.
- About 1/3 of the patients have bruises and hemorrhages.
- About 1/4 of the patients have severe infections involving the lungs, soft tissue, or skin.
- Splenomegaly and hepatomegaly are uncommon and are seen in<25% of the cases.
- Lymphadenopathy is even less common.
- Gingival hypertrophy or skin infiltration by the tumor occurs in 50% of the patients with monocytic leukemia.
- Acute promyelocytic leukemias are more commonly seen with severe bleeding from the DIC.
- Fever and fatigue are present in most of the patients.
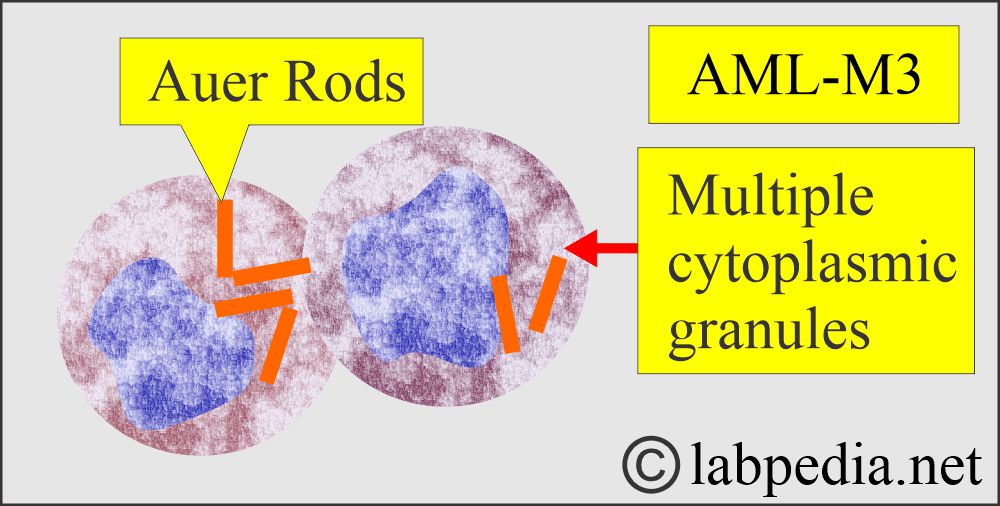
- Acute promyelocytic leukemia (M3) causes disseminated intravascular coagulopathy (DIC) and bleeding.
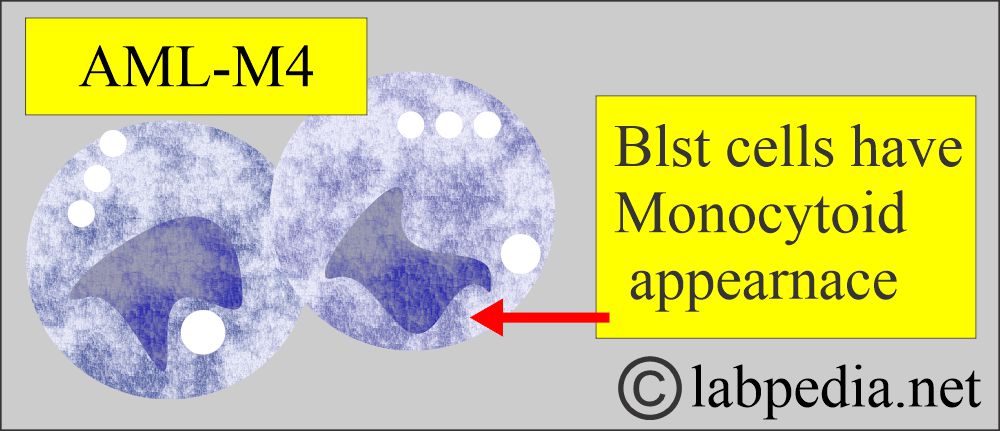
- Acute myelomonocytic leukemia (M4) and acute monocytic leukemia (M5) cause gum hypertrophy.
- Tumor infiltration of AML may be called granulocytic sarcoma (Chloroma); skin and bone, particularly the sternum, ribs, and orbit, are common sites. Myeloid sarcomas may involve many organs.
- These patients may have respiratory distress due to leukostasis within the pulmonary vasculature.
- There may be retinal hemorrhage due to thrombocytopenia.
How will you classify Acute myeloid leukemia (AML)?
French-American-British (FAB) Classification of Acute Myelocytic Leukemia:
- FAB has 85% concordance. This is based on morphology and cytochemistry.
- Immunophenotyping and cytogenetics provide additional information on the treatment.
- WHO criteria for the AML is ≥20% blast cells.
How will you describe the FAB classification of AML?
| FAB type | Characteristic features | Frequency |
|
Acute myeloblastic leukemia with minimal differentiation.
|
2% to 3% |
|
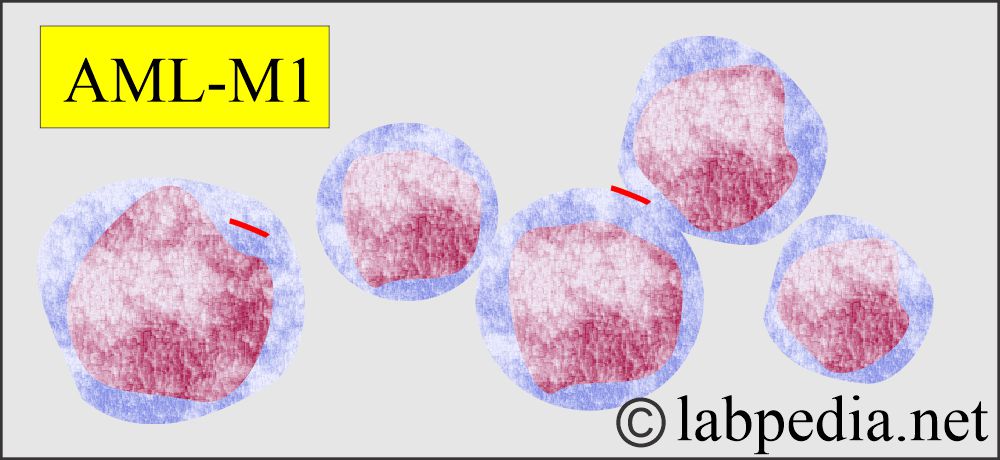
Acute myeloblastic leukemia without differentiation.
|
20% |
|
Also called Acute myeloid leukemia (AML) with maturation.
|
25% to 30% |
|
Acute Promyelocytic Leukemia.
|
5% to 10% (8% to 15%) |
|
Acute Myeloid Monoblastic Leukemia (Acute myelomonocytic leukemia).
|
20% to 30% (20% to 25%) |
|
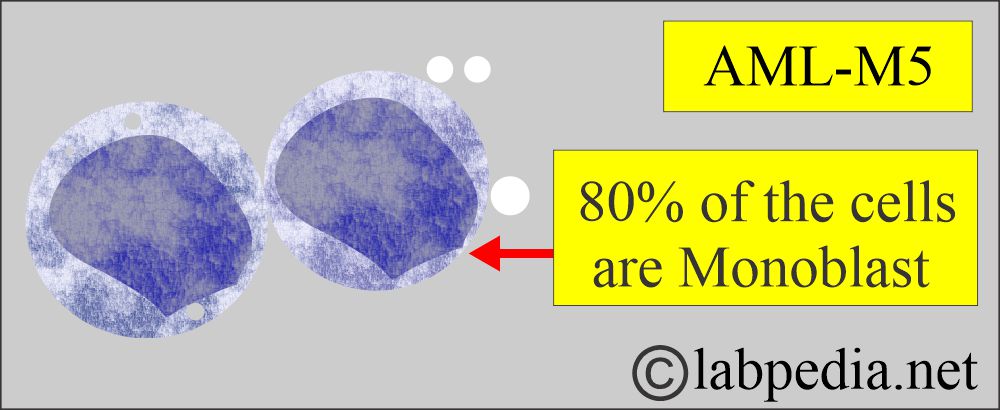
Acute Monoblastic Leukemia.
|
10% |
|
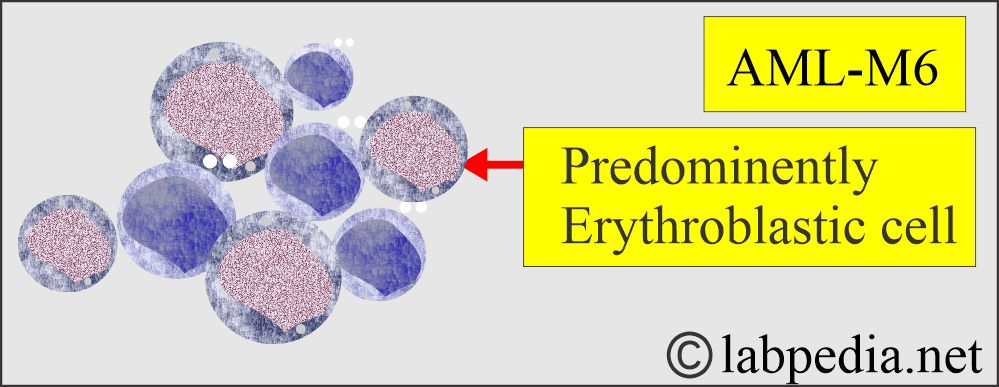
Acute Erythroleukemia.
|
5% |
|
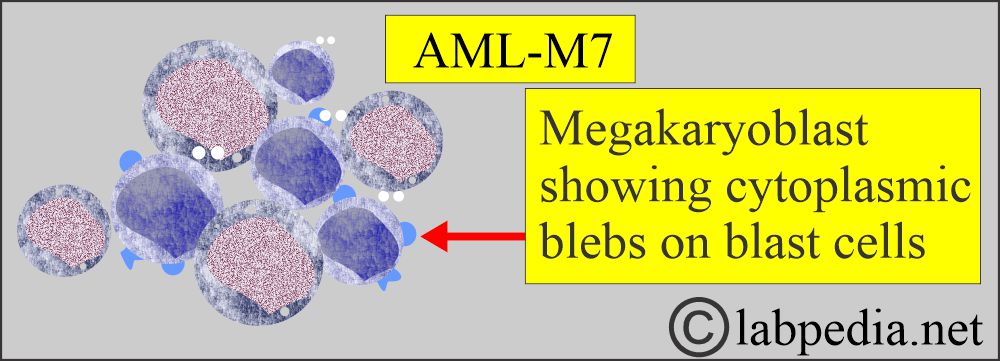
Acute Megakaryoblastic leukemia.
|
1% to 2% (1%) |
How will you summarize the FAB classification of acute myelocytic leukemia?
| Abbreviation of FAB | Differentiating features of AML cells | Type of leukemia |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
How will you diagnose Acute myeloid leukemia (AML):?
- WBC count is variable.
- 25% of the patients have>50,000/cmm.
- 25% has <5000/cmm, and 5% to 10% has count between 5000 to 10,000/cmm.
- A count>100,000/cmm is reported in <10% of the patients, but these patients have severe CNS or respiratory diseases due to leukostasis.
- In these patients, leukapheresis and chemotherapy may be life-saving.
- WBC morphology. These are morphologically abnormal and immature cells.
- 20% or more of the blast cells in the bone marrow are diagnostic of AML.
- Ther blast cells of AML are larger than lymphoblast cells and have more significant heterogeneity in size and shape.
- AML blast cells are most abundant in the cytoplasm and often contain cytoplasmic granules.
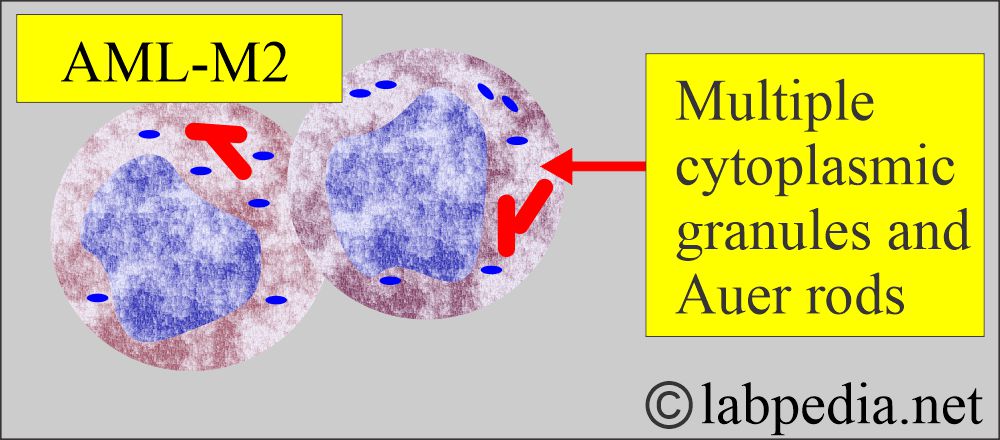
- About 10% of the AML cases show Auer rods and azurophilic granules with Wright’s stain.
- The peripheral blood smear may show blast cells in 85% to 90% of the cases.
- FAB divides Acute myelocytic leukemia (AML) into:
- M1, M2, and M3 with granulocytic differentiation.
- M4, M5 with at least 20% monocytoid appearance.
- M6 with a high proportion of erythroblasts.
- M7 with predominantly megakaryoblasts.
- M0 with minimal myeloid differentiation. This needs immunohistochemical stains for the diagnosis. These cases have myeloid antigens on their surface and negative myeloperoxidase activity.
- Biochemical tests:
- In 50% of the cases, uric acid is raised, which indicates increased cell turnover.
- Lactate dehydrogenase (LDH), about 50%, shows a raised level. This is not as common as in ALL.
- Bone marrow shows >20% blast cells. Promyelocytes are numerous, particularly in M3.
- Auer rods can be seen in the cytoplasm.
- Cytochemical stains, peroxidase reaction, and Sudan black are positive.
- Nucleated RBCs may be seen.
- Platelet count may be low and may see a count between 30,000 to 100,000/cmm.
- In some cases may be <20,000/cmm.
- Coagulation abnormalities are also seen. PT, PTT, and Thrombin time are prolonged.
How will you treat Acute myeloid leukemia (AML)?
- There is a supportive and specific treatment for AML.
- Stem cell transplantation and autologous transplantation may be performed. However, because of the toxicity, there is no overall benefit.
- The treatment of patients over 70 years of age with AML is poor because of primary disease resistance and poor tolerability of intensive therapy.
- Death in older adults is usually from hemorrhage, infections, and failure of the heart, kidneys, or other organs compared to younger patients.
What is the prognosis of Acute myeloid leukemia (AML)?
- 50% of children and young adults may have a long-term cure.
- Recently, with the newer advanced chemotherapy, the prognosis in the 60 to 70 years is better.
- Cytogenetics abnormalities and initial response to the treatment are the major predictors of the prognosis.
- AML complete remission is 60% to 70%, and disease-free survival is 20% to 40%.
- <60 years of the patients, complete remission is 70% to 80%, and disease-free survival is 40%.
- >60 years of complete remission is 45% to 55%, and disease-free survival is 5% to 10%.
What are the prognostic factors for Acute myeloid leukemia (AML)?
| Clinical parameters | Good prognosis | Poor prognosis |
|
|
|
|
|
|
|
|
|
|
|
|
Acute Lymphoblastic Leukemia (ALL)
What is the common age for Acute lymphoblastic leukemia (ALL)?
- ALL constitute 20% of adult leukemia, which is more common in children.
- In adults, 80% of the ALL are B-cells, and 20% are T-lymphocytes origin.
- This is most common in childhood.
- It mostly occurs before the age of 4, peaking between 2 and 10 years; it is also called childhood leukemia.
- ALL comprises >80% of childhood leukemia.
- 90% have chromosomal abnormalities.
- Down’s syndrome has a 15 times higher incidence of ALL.
- These are rare after the age of 30 years.
- These are most common in children and young adults.
- The second peak may be seen in the middle and old age groups.
- L3 leukemia is most common in developing countries and may be associated with infection by the Epstein-Barr virus in younger people.
- Children with trisomy 21 (Down’s syndrome) have an increased risk for childhood acute lymphocytic and acute myelocytic leukemias.
- Immunodeficiency diseases like ataxia-telangiectasia, osteogenesis imperfecta, siblings of ALL, and Poland syndrome have an increased incidence.
What are the signs and symptoms of Acute lymphoblastic leukemia (ALL)?
- S/S of acute AML and ALL are similar, except the onset of ALL is strikingly acute.
- The onset is sudden. There is no preleukemic stage.
- Symptoms are present only for a few weeks before the diagnosis.
- There is fatigue and fever. There is malaise, lethargy, and weight loss.
- There may be bleeding and infections.
- Enlargement of lymph nodes, spleen, and liver is more common than AML, affecting 50% of adults.
- Central nervous system (CNS) involvement may be present in 5% to 10% of the cases.
- This shows leukemic meningitis.
- Cranial nerve palsy is more common in the 6th and 7th nerves.
- There are headaches and papilledema due to meningeal involvement and the obstruction of the outflow of CSF.
- CSF shows increased protein and leukemic cells.
- Bone pain is due to the infiltration of the leukemic cells.
- Retinal hemorrhage is due to thrombocytopenia.
- Chest X-ray shows a thymic mass in 10% to 15% of the adults.
How will you Classify Acute lymphoblastic leukemia (ALL)?
- Classification of ALL is based on cytological features like:
- Cell size.
- Nuclear chromatin pattern.
- Nuclear shape.
- Presence of nucleoli.
- Amount of basophilia in the cytoplasm.
- 80% of the ALL are B-lymphocytes in origin.
- 15% to 20% of cases arise from T-lymphocytes. These cells will express CD2, CD5, and CD7.
- T- L ALL are lacking CD19 and CD20.
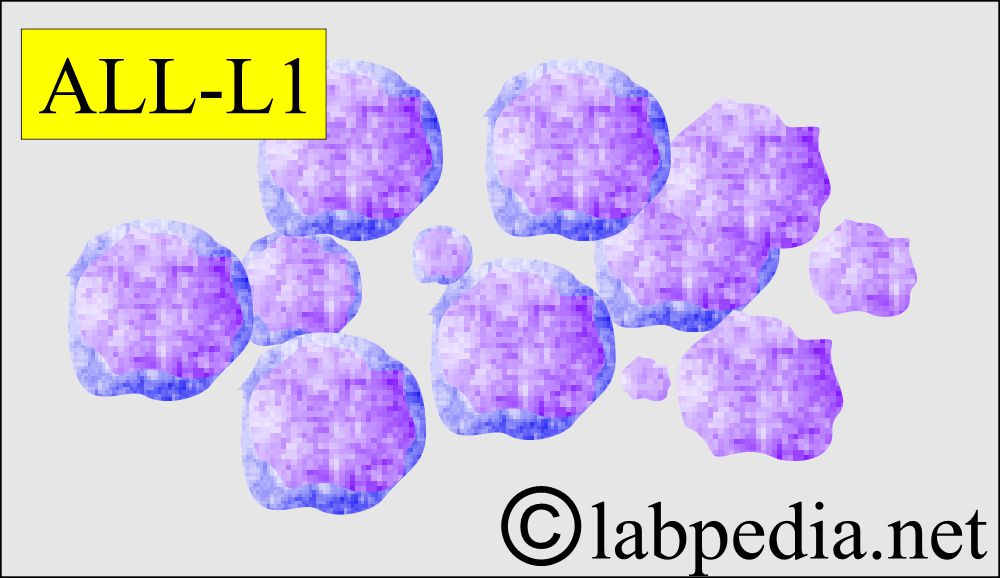
- L1 – ALL accounts for more than 80% of cases in children and consists of predominantly small lymphocytes, up to twice the size of small lymphocytes.
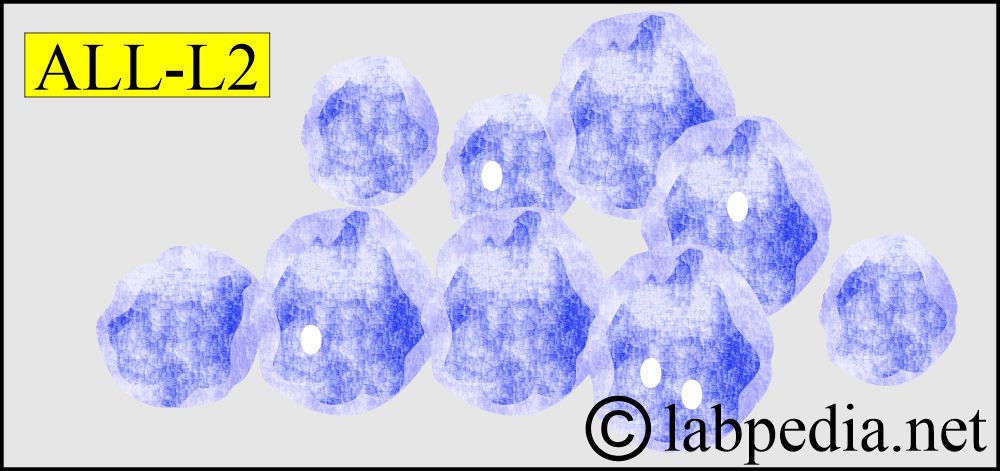
- L2 – ALL accounts for the majority of adult leukemia. The cells are larger than those in L1 and often heterogeneous in size.
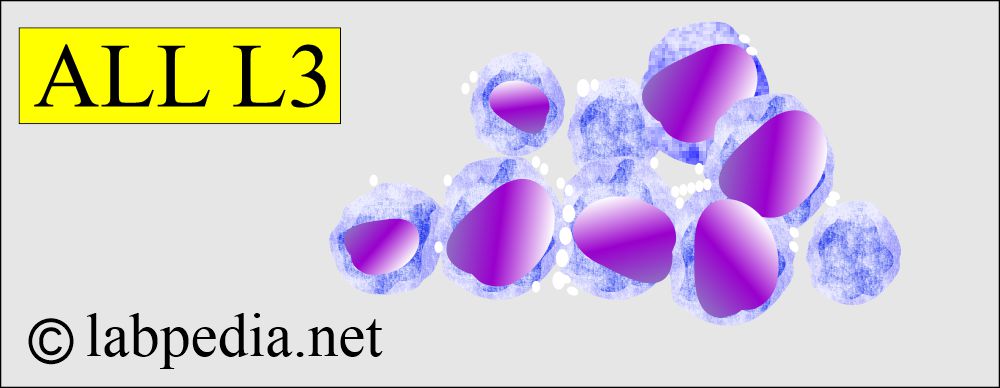
- L3 – ALL is the least common form in approximately 3% to 4% of children and adults. The cells are morphologically identical to those in Burkitt’s lymphoma.
- FAB is no longer used.
Please discuss the FAB (French-American-British) classification of Acute Lymphocytic Leukemia?
|
|
|
|
|
|
How will you classify Acute lymphoblastic leukemia based on the Cytochemical method?
| Type of leukemia | Periodic acid-Schiff reaction (PAS) | Acid phosphatase reaction (AP) | Terminal deoxynucleotidyl transferase reaction (TdT) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What are the lab findings of acute lymphoblastic leukemia (ALL)?
- Lab diagnosis depends upon the following:
- Morphology.
- Cytochemistry.
- Immunophenotyping.
- Genetic analysis.
- WBC count is variable from very high to low count.
- There are anemia and thrombocytopenia.
- Bone marrow: Very few leucocytes are seen.
- Difficult to find normal WBCs or RBCs in the bone marrow.
- Characteristically, there are blast cells. >50% are lymphoblastic cells.
- Auer rods are absent.
- Serum LDH, uric acid, and ESR are often raised.
- Flow cytometry studies (differentiation of the antigens over the surface of these abnormal cells) provide rapid diagnosis.
- 80% of the ALL arises from the B-lymphocytes and will show CD molecules of CD19, CD20, or both.
- A few ALL cases may show CALA (common ALL antigen), which is CD10.
- Roughly 20% of the CD10-positive cases may show cµ and are called pre-B – ALL.
- Special stains for Acute lymphoblastic leukemia (ALL):
- Sudan’s black and peroxidase reactions must be negative to support the diagnosis of ALL.
- PAS stain shows clumpy positivity by glycogen in all ALL except the L3, which is negative.
- Cloroectate esterase and lysozyme stains were negative in all ALL.
- α-naphthyl acetate esterase may be positive in T-lymphoblast.
- All ALL cases contain the TdT (terminal deoxynucleotidyl transferase) enzyme, which is a fairly reliable marker of the ALL.
- ALL—L3 often stains with oil red O because of the neutral lipids present in the vacuoles.
- What is the morphology of the cells?
- L1
- Small uniform blast cells
- L2
- Larger and more variable sizes of the cells
- L3
1. Uniform cells with basophilic and sometimes vacuolated cytoplasm, typical of Burkitt’s lymphoma.
2. Philadelphia chromosomes-positive in acute lymphoblastic leukemia and t(8;14) found.
How will you treat Acute lymphoblastic leukemia (ALL)?
- The aim is to restore the normal function of the bone marrow with multiple chemotherapy drugs.
- Typically, four drugs, e.g., vincristine, prednisone, daunorubicin, L-asparaginase, and cyclophosphamide, are used for induction and remission.
- 80% to 90% of adults enter complete remission.
- Around 30% to 50% will be alive and disease-free for three years.
What is the prognosis of Acute lymphoblastic leukemia (ALL)?
- Patients with a count >30,000/cmm have a shorter duration of remission than patients with a lower count.
- Older age >60 years is not a good favorable factor.
- Another factor that matters in the prognosis is:
- Circulating blast cells %.
- The degree of bone marrow involvement.
- The presence of splenomegaly, hepatomegaly, and lymphadenopathy.
- LDH level.
- Involvement of the CNS at the time of diagnosis.
- The time needed to get the remission is 4 to 6 weeks.
- Ph1chromosome is present in ≤25% of adults and 3% of children. Its positivity shows poor prognostic signs.
- Overall, in acute lymphoblastic leukemia (ALL), 80% to 90% have complete remission, and disease-free survival is 30% to 40%.
- T-ALL 90% to 95% have complete remission, and disease-free survival is 60% to 65%.
- Precursor B-Cell ALL has 75% to 85% complete remission, and disease-free survival is 30% to 40%.
- ≥60 years of complete remission of 75% to 80%, and disease-free survival is 10% to 25%.
- Poor prognostic signs are:
- Platelets count <50,000/cmm.
- WBCs count >100,000/cmm.
- Pre-B phenotype.
- Cytogenetic abnormalities.
- CD10 seronegative type.
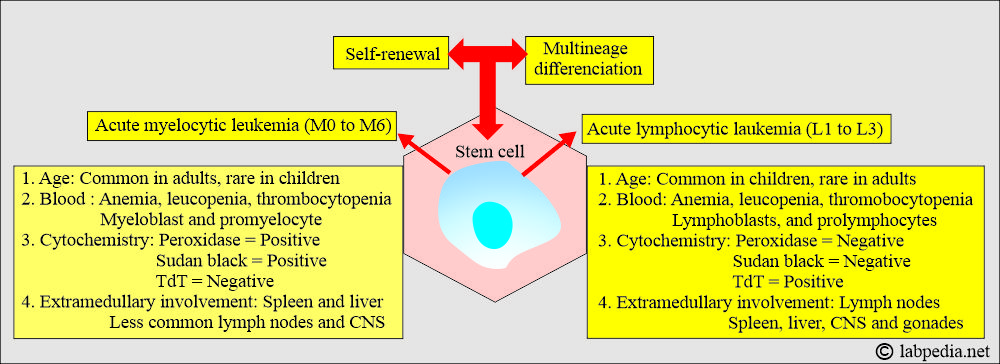
What is the difference between Acute Myelocytic Leukemia and Acute Lymphocytic Leukemia?
| Characteristic Features | Acute Myelocytic Leukemia | Acute Lymphocytic Leukemia |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What is the prognosis of Acute lymphoblastic leukemia (ALL) and Acute myeloid leukemia (AML)?
| Type of leukemia | Complete remission | Disease-free survival |
| ALL |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| AML | ||
|
|
|
|
|
|
|
|
|
|
|
|
What is the value for layman?
- A blood examination is advised if the patient has a high TLC.
- If the patient has anemia.
- If the patient has enlarged lymph nodes.
- In the case of patients with fever and weakness,
Questions and answers:
Question 1: Which type of acute myeloid leukemias has frequent Auer rods?
Question 2: Is Sudan black stain positive in Acute lymphoblastic leukemia?

Thanks
Thanks for your comment
very informative and concise
Thanks for the comments.
Pharm line is going down
THANK YOU SO MUCH DR. RIAZ
I am in medical laboratory technician school now. Your website is a great source to learn
Thanks.
Thanks a lot sir
thanks from your information your website is really nice and
Thanks.