Acid-base Balance:- Part 3 – Respiratory Acidosis and Alkalosis
Respiratory Acidosis and Alkalosis
What sample is needed for Respiratory Acidosis and Alkalosis?
- The better choice is the Radial artery.
- The sample may be taken from the femoral artery or brachial.
- It can be drawn from the indwelling arterial line.
- The tests are done immediately because oxygen and carbon dioxide are unstable.
- Place the sample on ice and immediately transfer it to the lab.
- Arterial blood is better than venous blood.
- For venous blood, a syringe or tube, be filled, and a tourniquet for a few seconds.
- Arterial blood is risky, and a trained person should do it.
- Never apply a tourniquet.
- Don’t apply the pull to the plunger of the syringe.
How will you differentiate between arterial vs. venous blood?
Arterial blood (ABG):
- Arterial blood (ABG) gives a good mixture of blood from various body areas.
- Arterial blood color is bright red.
- Arterial blood measurement gives a better status of lung oxygenation.
- If arterial O2 concentration is normal, it indicates lung function is normal.
- If mixed venous O2 concentration is low, the heart and circulation fail.
- Arterial blood gives information about the lung’s ability to regulate the acid-base balance through the retention or release of CO2.
- It can check the effectiveness of the kidneys in maintaining the appropriate bicarbonate level.
Venous blood (VBG)
- It gives information about the local area from where the blood sample is taken.
- Venous blood color is dark red.
- Metabolism of the extremity varies from area to area.
- In shock, the extremities are cold, and less blood perfusion.
- During the local exercise of the extremities, such as opening and closing the fist with power.
- In case there is an infection of the sample area.
- A blood sample from the central venous catheter is not a good mix of blood from various body parts. For well-mixed blood sample should be taken from the right ventricle or the pulmonary artery, which is not an easy procedure.
- A blood sample from the central venous catheter:
- Shows low O2 concentration, which means that:
- Either the lungs have not oxygenated the arterial blood well.
- Or the Heart is not circulating the blood effectively.
- Shows low O2 concentration, which means that:
Summarize the difference between arterial and venous blood?
| Biochemical parameters | Arterial blood | Venous blood |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What are the precautions for the collection of blood?
- Avoid pain and anxiety in the patient, which will lead to hyperventilation.
- Hyperventilation due to any cause leads to decreased CO2 and increased pH.
- Keep blood cool during transit.
- Don’t clench your finger or fist. This will lead to lower CO2 and increased acid metabolites.
- pCO2 values are lower in the sitting or standing position than in the supine position.
- Don’t delay the performance of the test.
- Avoid air bubbles in the syringe.
- Excess of heparin decreases the pCO2, maybe 40% less.
- Not mixing the blood properly before the test may give a false result.
- A prolonged tourniquet or muscular activity decreases venous pO2 and pH.
- The best way to collect arterial or venous blood is anaerobic.
- Arterial blood precautions:
- Only syringe and needle, no tourniquet, no pull on the plunger.
- Venous blood precautions:
- The heparinized evacuated tube’s needle and a syringe filled, drawn a few seconds after the tourniquet.
- Liquid heparin is the only suitable anticoagulant with the proper amount.
- Less amount will lead to clot formation.
- The increased amount will lead to increased CO2 and a decrease in pH.
- This will lead to a dilutional error.
- Glass collection devices are better than plastic.
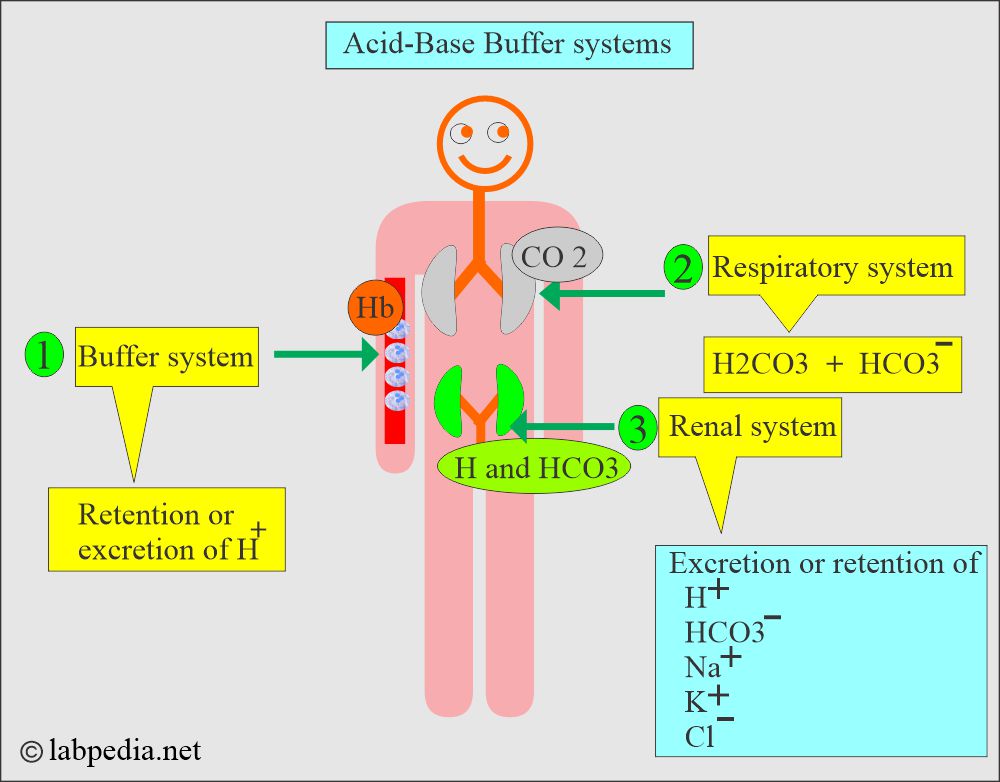
How will you define acid-base disturbance and control?
- H+ ions and electrolytes disturbances may be:
- Acute.
- Chronic.
- Modest or severe.
- Simple or mixed.
- When there is an accumulation of H+ ions, it is called acidosis.
- When blood pH declines below 7.3, this process is called acidemia.
- When there is a deficiency of H+ ions, it is called alkalosis.
- Blood pH rises above 7.45 is called alkalemia.
- Conditions related to the respiratory system lead to respiratory acidosis or alkalosis.
- There are metabolic conditions related to the kidneys, and abnormal intake/output leads to metabolic acidosis/alkalosis.
- The blood pH is normally maintained at 7.38 to 7.42.
- Any deviation from this range indicates a change in the concentration of H+ ions.
- Blood pH is a negative logarithm of [H+], as shown in the following equation:
- pH = log10 [H+]
- This equation shows that an increase in the H+ ions will lead to a fall in the blood pH, which is called acidemia.
- So, a decrease in the H+ ions will lead to an increase in the pH of the blood, which is called alkalemia.
- The conditions that cause the pH change are called acidosis and alkalosis.
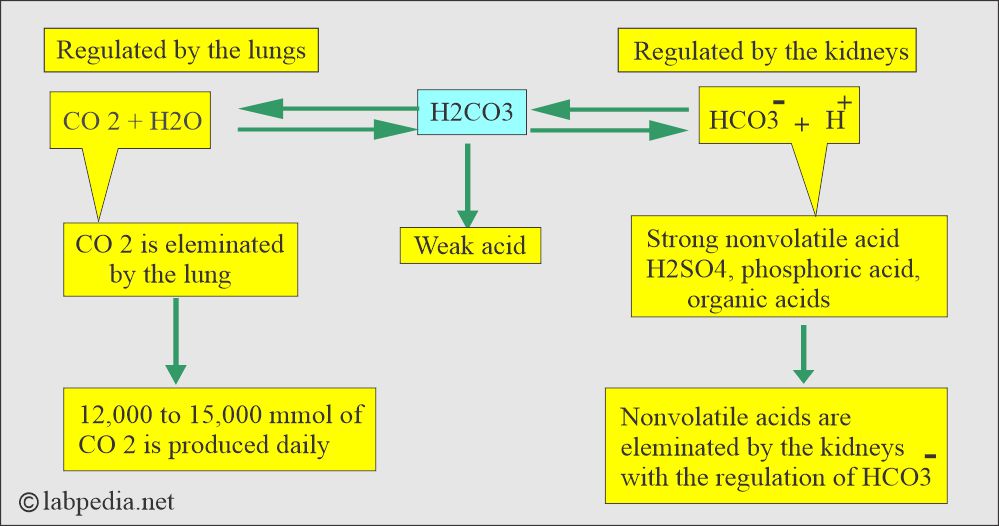
- The following diagram explains how pH is maintained by the arterial carbon dioxide tension (pCO2) and plasma bicarbonate (HCO3–).
- Plasma HCO3– decrease in the plasma caused by gastrointestinal or renal losses will increase H+ ions and lowers the pH.
What are the indications for the diagnosis of respiratory Alkalosis/acidosis?
- In the case of chronic lung disease.
- Cardiopulmonary arrest.
- Sleep apnea.
- Myasthenia gravis.
- Laryngospasm.
- Chronic obstructive pulmonary disease.
Respiratory acidosis
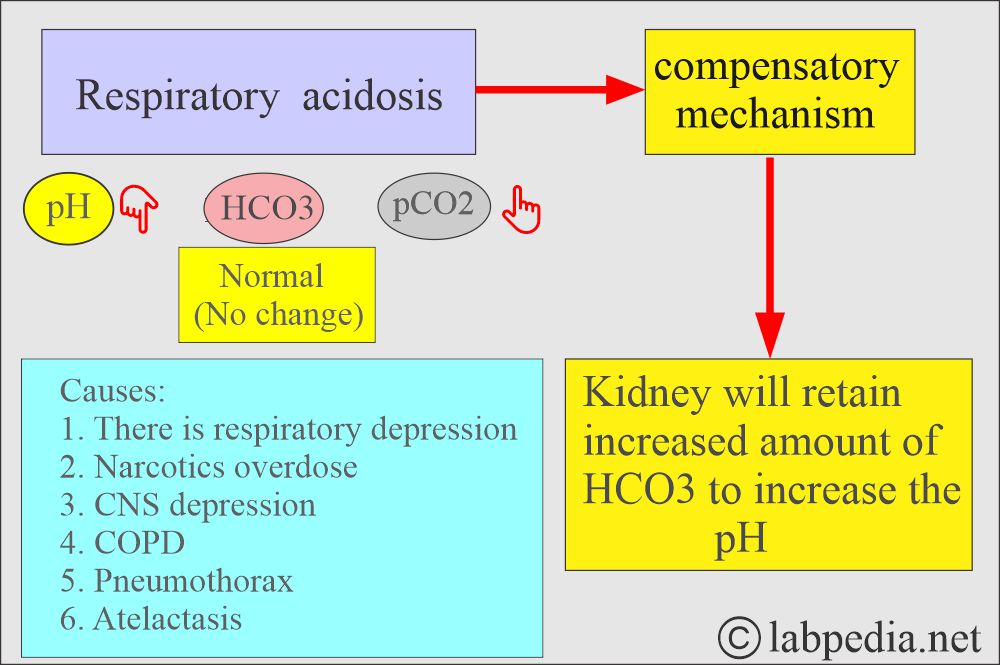
How will you define respiratory acidosis?
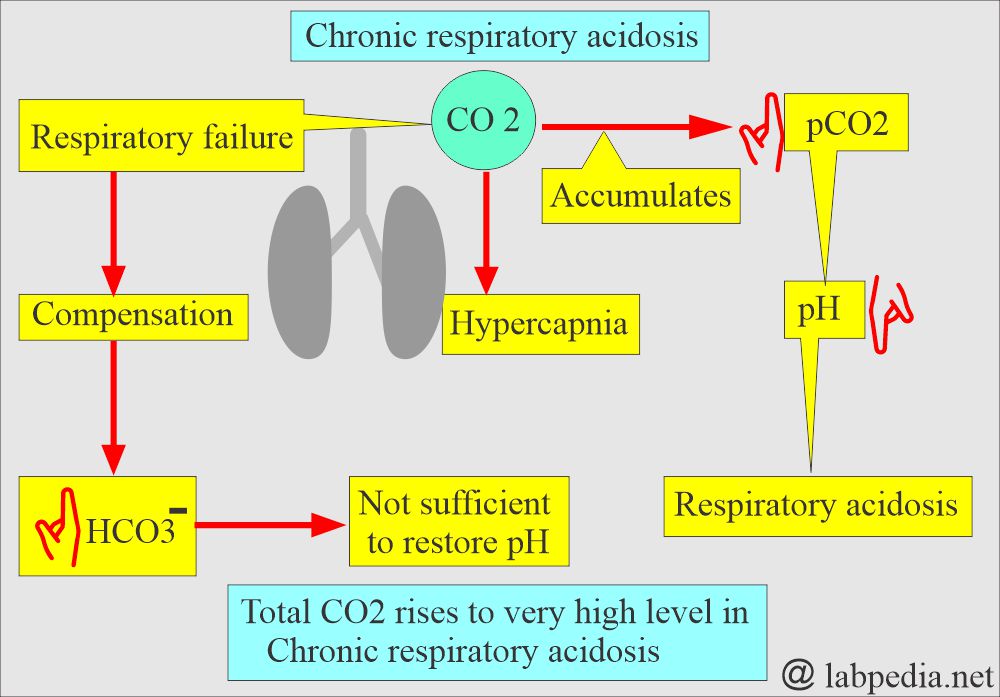
- With respiratory failure, CO2 accumulates (hypercapnia).
- This state will raise the pCO2, which causes the pH to drop and leads to acidosis.
- This is a decrease in alveolar ventilation in relation to the metabolic production of CO2, which produces respiratory acidosis by increasing carbonic acid.
How will you discuss the pathophysiology of respiratory acidosis?
- Alveolar ventilation provides the necessary oxygen for oxidative metabolism and eliminates the CO2 produced by these metabolic processes.
- There is a depression in the ventilation, resulting in excess CO2 (hypercapnia) in blood circulation.
- A decrease in alveolar ventilation in relation to the metabolic production of CO2 produces respiratory acidosis through an increase in H2CO3 acid.
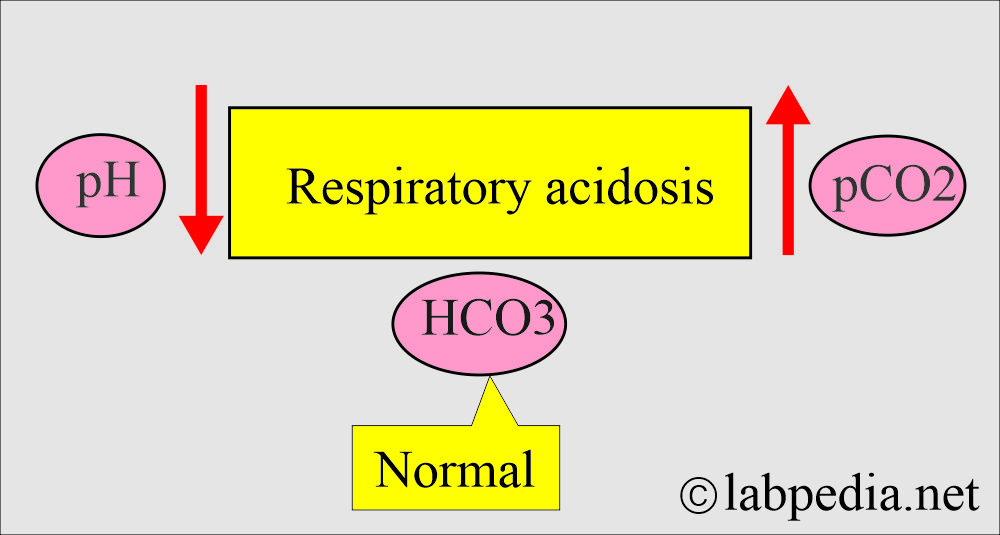
- The arterial CO2 tension (or pressure) PaCO2 is >45 mm Hg.
- This is seen in respiratory failure, where CO2 accumulates, called hypercapnia.
- This condition will raise the pCO2 and cause the pH to drop.
- The HCO3– will increase to compensate, but this is insufficient to restore the pH to a normal level.
- CO2 level rises, and this retained CO2 combines with water and forms H2CO3.
- H2CO3 dissociates to release H+ and HCO3– ions.
- Increased paCO2 and free H+ ions stimulate the medulla to increase the respiratory rate and expel the CO2.
- As the pH falls to 2.3, diphosphoglycerate accumulates in the RBCs, altering the Hb (hemoglobin) to release the O2 (oxygen).
- Hb picks up H+ ions and CO2 and removes both from blood circulation.
- If the respiratory mechanism fails, rising paCO2 stimulates the kidneys, retains HCO3– and Na+ (sodium) ions, and starts excreting H+ ions.
- Total CO2 may rise to a very high level of chronic respiratory acidosis.
What are the signs and symptoms of respiratory acidosis?
- There is often breathlessness.
- The patient is restless.
- There is a headache, dyspnoea, and tachypnea.
- There is apprehension followed by lethargy.
- The patient will have disorientation.
- There are muscle twitching and tremors.
- The skin will be warm and flushed due to raised CO2, which causes vasodilatation.
- There may be hypertension or hypotension.
- There are atrial and ventricular arrhythmias.
- The patient will have convulsions and ultimately go into a coma.
What are the causes of respiratory acidosis?
- Acute respiratory acidosis:
- This occurs with sudden obstruction to:
- The airway.
- Chest trauma damages the respiratory muscles.
- Acute paralysis or depression of the CNS respiratory center.
- HCO3– rises 1 meq/L for each 10 mmHg rise in pCO2.
- This occurs with sudden obstruction to:
- Chronic respiratory acidosis:
- This chronic respiratory acidosis is difficult to treat compared to acute respiratory acidosis.
- This will take place by:
- Chronic obstructive pulmonary diseases include bronchitis, emphysema, or scarring.
- Accumulation of CO2 lasting days, weeks, or months will provoke a sustained increase in HCO3—generation and lead to enhanced renal excretion of the H+ ions with chronic CO2 retention.
- HCO3– rises 3.5 meq/L for each 10 mm Hg rise in pCO 2.
- The serum level of Na+ and K+ may be normal or mildly raised.
- Suppression of the medullary respiratory center:
- Sleep apnea.
- Sedation medicines.
- Cardiopulmonary arrest.
- Upper respiratory obstruction:
- Laryngospasm.
- Aspiration of the foreign body or vomitus.
- Obstruction in sleep apnea.
- Defective respiratory muscle function:
- Myasthenia gravis.
- Guillain-barre syndrome.
- Botulism.
- Hypokalemia (severe).
- Poliomyelitis.
- Myxedema.
- Amyotrophic lateral sclerosis.
- Defect in the pulmonary gas exchange:
- Acute respiratory distress syndrome.
- Pneumothorax.
- Hemothorax.
- Severe asthma.
- Severe pneumonia.
- Chronic obstructive pulmonary disease.
How will you diagnose respiratory acidosis?
- pH = <7.35 to 7.45.
- paCO2 = >45 mm Hg.
- HCO3– = Normal (in the acute stage).
- HCO3– = Increased (in the chronic stage).
How will you treat respiratory acidosis?
- Treatment of the pulmonary causes:
- If there is obstruction by the foreign body, remove that immediately.
- Mechanical ventilators may be needed.
- Give bronchodilators.
- If there is pneumonia, then start antibiotics.
- If there is pneumothorax, then put a chest tube.
- In the case of pulmonary embolism, thrombolytic and anticoagulants should be started.
- Remove the secretions by bronchoscopy.
- Treatment of chronic obstructive pulmonary disease (COPD):
- Give O2 at a slow rate.
- Start bronchodilators.
- Start corticosteroids.
- You can also give I/V sodium bicarbonate.
- Other drugs are needed to treat the cause.
Respiratory Alkalosis
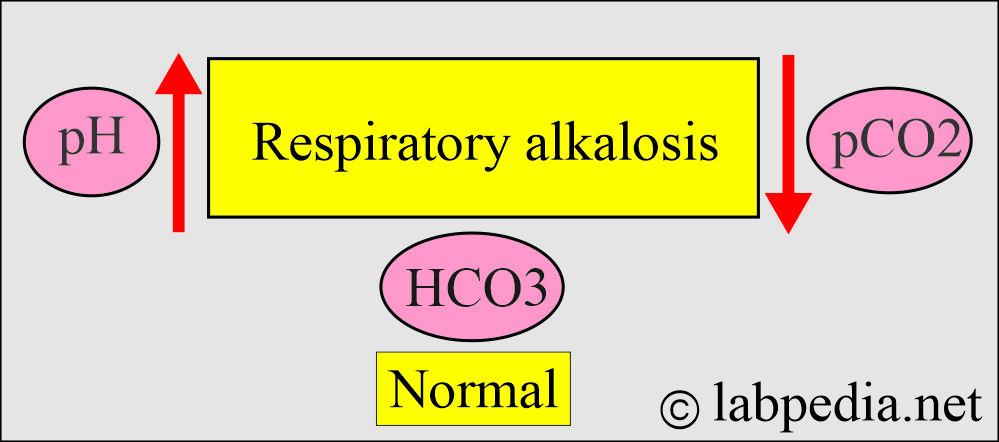
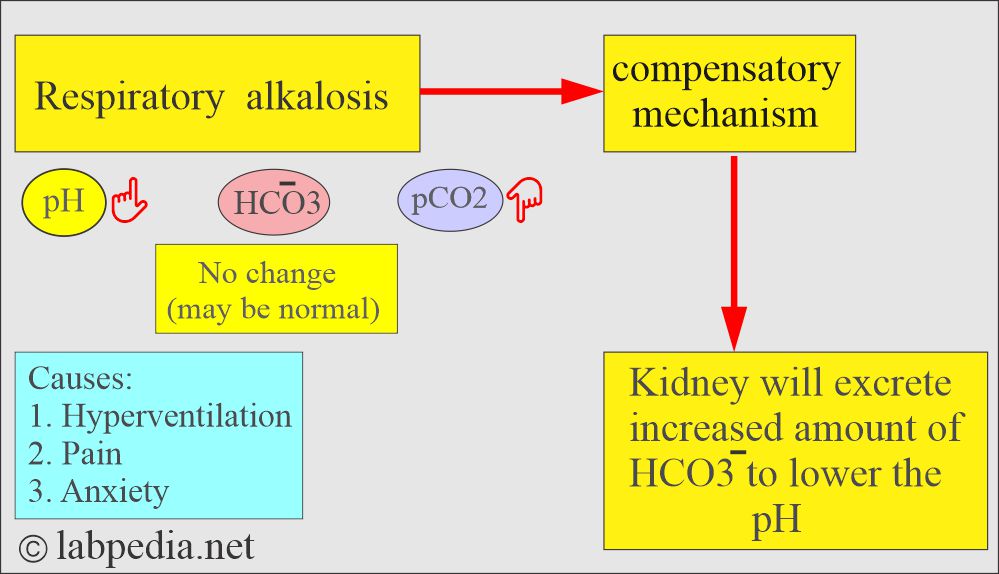
How will you define respiratory alkalosis?
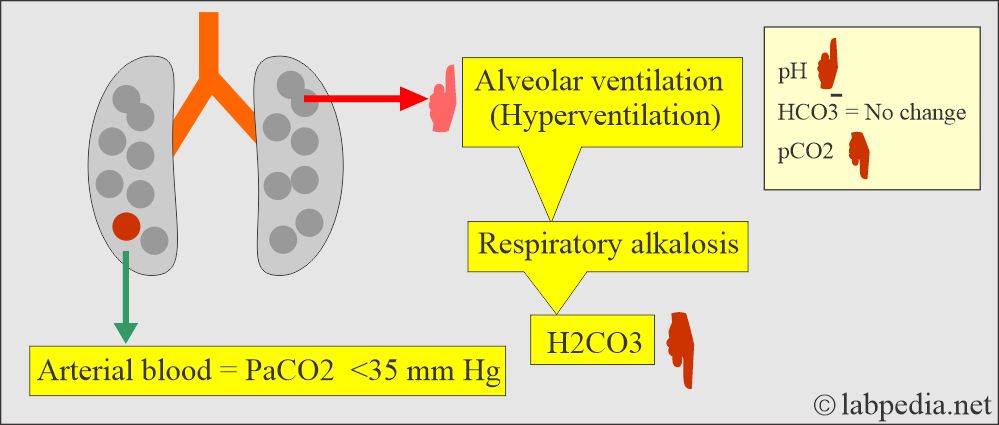
- This is due to over-breathing, causing excessive CO2 excretion, leading to a rise in blood pH.
How will you discuss the pathophysiology of respiratory alkalosis?
- Overbreathing causes excessive CO2 to be exhaled and causes the blood pH to rise.
- Acute respiratory alkalosis interacts with intracellular and protein buffers before affecting the HCO3– system.
- After the adjustment, blood HCO3– drops 5 meq/L for every 10 mmHg decline in pCO2.
- Alkalosis causes plasma proteins to have a more negative charge that, in turn, binds more ionized Ca++.
- This hypocalcemia increases neuromuscular excitability and leads to tetany.
- Respiratory alkalosis occurs when alveolar hyperventilation and an excessive reduction in plasma CO2 levels occur. This is called hypocapnia.
- In the case of initial hypoxemia, increased ventilation is mostly mediated by the chemoreceptors in the carotid body; these are located near the carotid artery’s bifurcation.
- Kidneys compensate by decreasing H+ excretion and HCO3¯ reabsorption.
- The PaCO2 is <35 mm Hg.
What are the causes of respiratory alkalosis?
- Pulmonary diseases due to hypoxemia:
- Pneumonia.
- Pulmonary embolism.
- Pulmonary edema.
- High-altitude syndrome.
- Severe anemia.
- Congestive heart failure.
- Stimulation of the medullary (respiratory) center:
- Hepatic encephalopathy.
- Sepsis with fever.
- Salicylates toxication.
- Hyperventilation syndrome.
- Pregnancy when there is increased progesterone.
- Cerebrovascular accidents.
- Pontine tumors.
- Hypermetabolic conditions:
- Fever.
- Anemia.
- Thyrotoxicosis.
- Hysteria.
- Cirrhosis.
- Gram-negative sepsis.
- Pregnancy.
What are the signs and symptoms of respiratory alkalosis?
- The central and peripheral nervous system is stimulated, leading to:
- There is light-headedness or Dizziness.
- The patient may be agitated.
- Confusion.
- Tingling of the extremities appears first around the mouth and in the fingers and toes, called circumoral and peripheral paresthesia.
- There is a carpopedal spasm, twitching, and muscle weakness.
- Light-headedness and weakness may occur and progress to unconsciousness.
- Convulsions.
- Ultimately, the patient goes into a coma.
- Deep and rapid respirations are the primary symptoms that cause respiratory alkalosis.
How will you diagnose respiratory alkalosis?
- The blood pH is >7.42.
- Decreased pCO2.
- HCO3: H2CO3 = 20:0.5
- Decreased H2CO3 level.
- HCO3– = Normal in the acute stage.
- HCO3– = Less than normal in the chronic stage.
How will you treat respiratory alkalosis?
- If there is intoxication like salicylates, induce emesis or use gastric lavage.
- May need treatment for fever or sepsis.
- O2-therapy for acute hypoxemia.
- In the case of CNS disease, treat those diseases.
- Ask the patient to breathe in the paper bags.
- Ventilators are needed.
- Treatment is mostly not needed.
- It is important to diagnose the cause and treat the underlying disease.
What are the characteristic features of respiratory acidosis and alkalosis?
| Clinical condition | Etiology of the condition and S/S | pH (7.37 to 7.43) | HCO3– (19 to 25 meq/L) | pCO2 (38 to 42 mmHg) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What are the panic values?
| Clinical parameter | Panic value |
|
|
|
|
|
|
How will you summarize the parameters needed for the acid-base balance?
| Lab test | Importance |
|
This will tell:
|
|
This is the partial pressure of CO2, and it will tell:
|
|
This is the partial pressure of the O2 in the arterial blood and tells:
|
Questions and answers:
Question 1: What are the panic values.
Question 2: What are S/S of metabolic alkalosis.