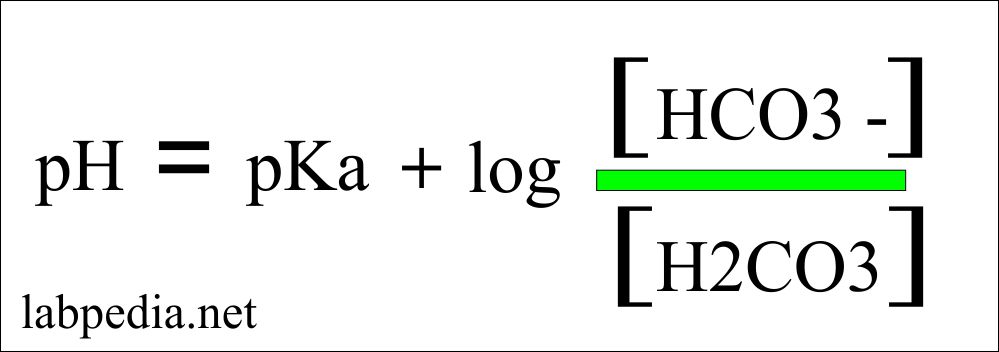Acid-Base Balance:- Part 1 B – Acid-Base System
June 1, 2023Uncategorized
Acid-base balance:
Sample of blood for acid-base balance
- pH and blood gases preferably should be done on arterial blood.
- Venous blood is not good for judging oxygenation.
- Venous blood can be used for acid-base status.
- Collect blood for electrolytes at the same time.
- Blood gases, electrolytes, and pH should be performed on blood specimens obtained at the same time because of the variation in blood gases which are labile.
Precautions for blood for acid-base studies
- The blood sample should be ice packed immediately.
- A delay of a few minutes will give false values.
The H+ ions concentration is commonly expressed as the pH.
- The buffering system becomes active in response to a change in the acid-base status.
- Buffers can absorb excessive H+ (acid) or OH‾ (base) without any
change in the pH. - The body normally maintains the arterial blood pH within a definite range of 7.35 to 7.45.
- The H+ ions concentration must be regulated within a narrow range for the body to maintain the body’s
normal functionality.- A slight change in the H+ concentration will change the body’s pH.
- H+ is needed for the :
- For the maintenance of cell integrity.
- It helps in the speed of the enzymatic reaction.
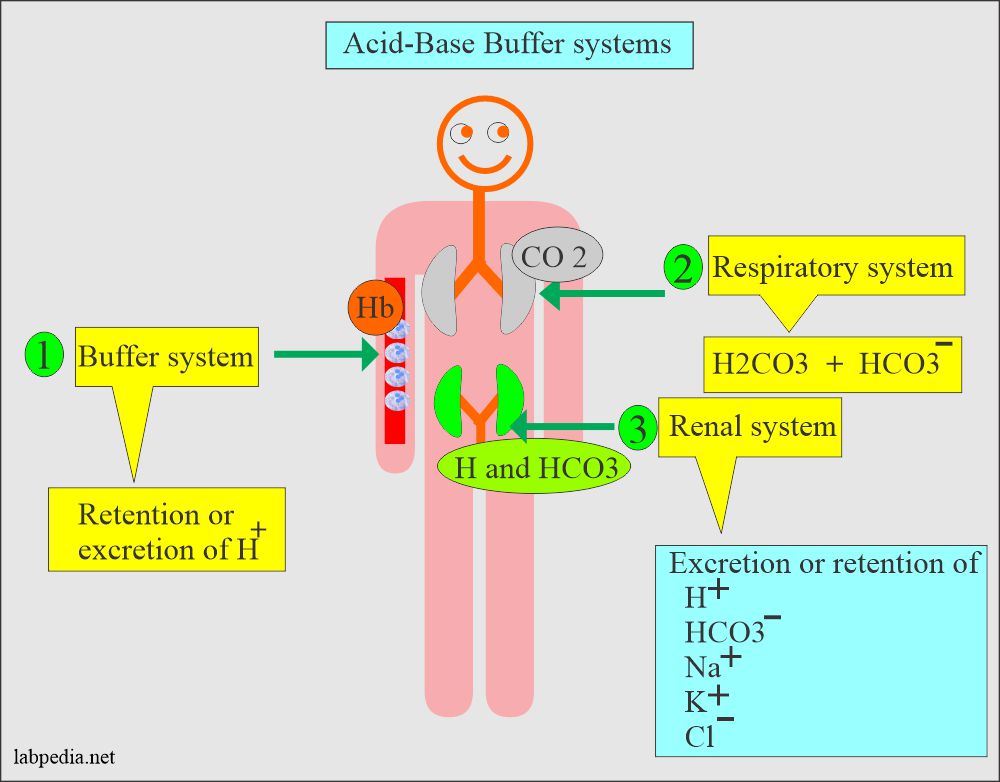
A buffer system of the body:
The buffer system:
- It works through the retention or excretion of the H+.
- Other minor buffer systems are phosphate and proteins.
The respiratory buffer system:
- It works through Carbonic acid-bicarbonate (H2CO3 / HCO3¯).
This will cover 80% of the pH control; the renal system does the rest. - There is a ratio of one part of Carbonic acid (H2CO3) and twenty parts of bicarbonate.
(HCO3−).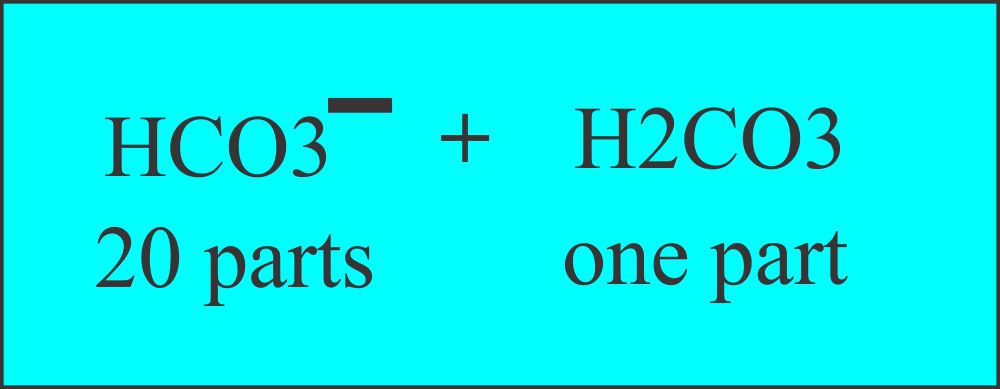
Buffer system, carbonic acid, and bicarbonate
- The carbonic acid level can be measured indirectly by measuring the pCO2 level.
- The lung controls the pCO2.
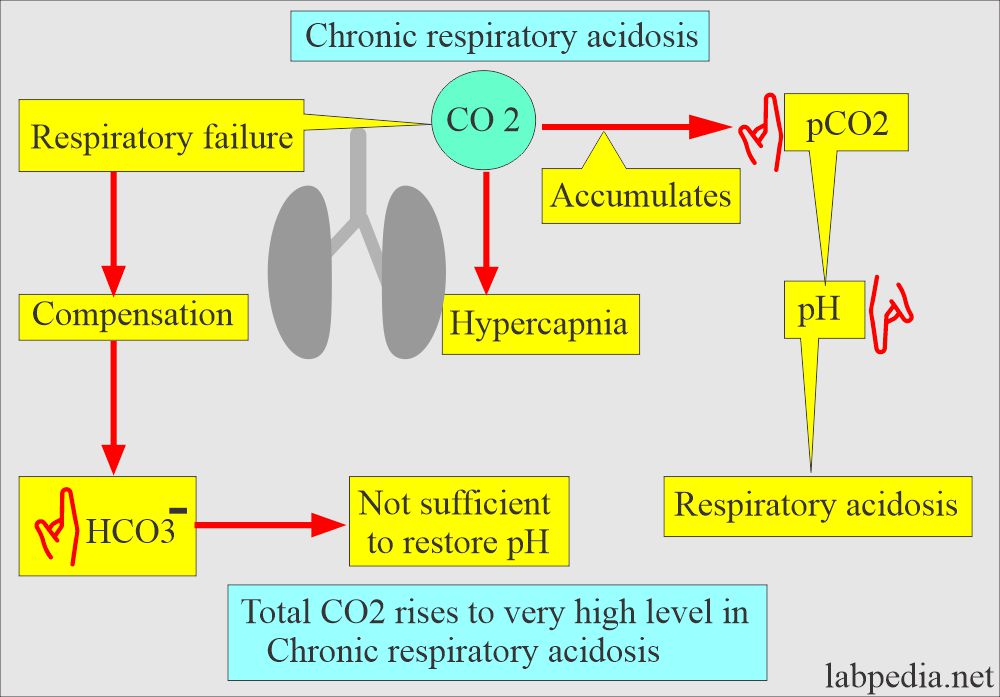
- More CO2 retained and more H2CO3 will lead to acidosis.
- Less CO2, and there will be fewer H2CO3, will lead to alkalosis.
- The respiration process supplies oxygen to the tissues and removes the carbon dioxide produced by cellular
metabolic activity. - External respiration:
- Where oxygen in the air is exchanged at the alveolar level with carbon dioxide in the blood.
- Internal respiration:
- Take place at the tissue level, where oxygen in the blood is delivered to the cells, and
carbon dioxide is transferred from the cells to the blood for disposal.
- Take place at the tissue level, where oxygen in the blood is delivered to the cells, and
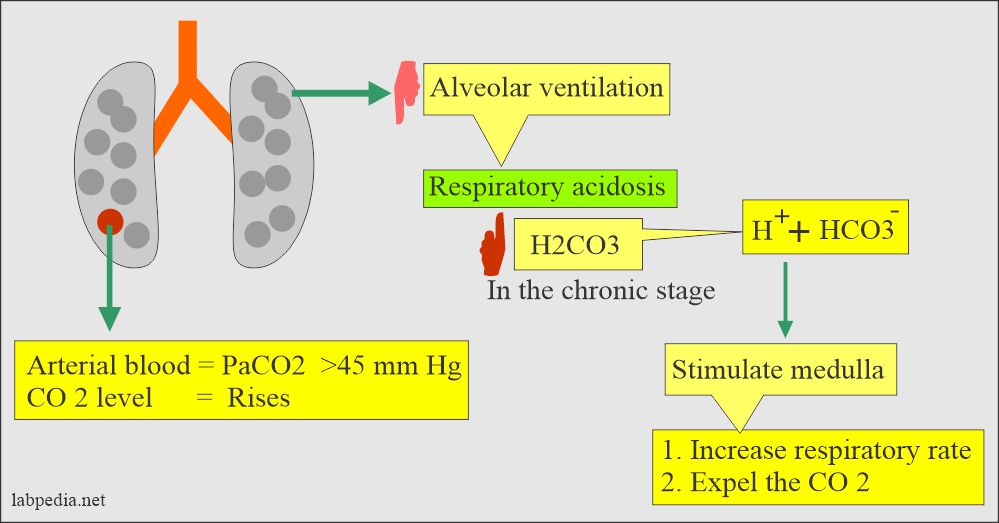
Role of lungs in case of Respiratory acidosis
- The medullary center, the brain stem, controls respiration by increasing CO2 and decreasing O2.
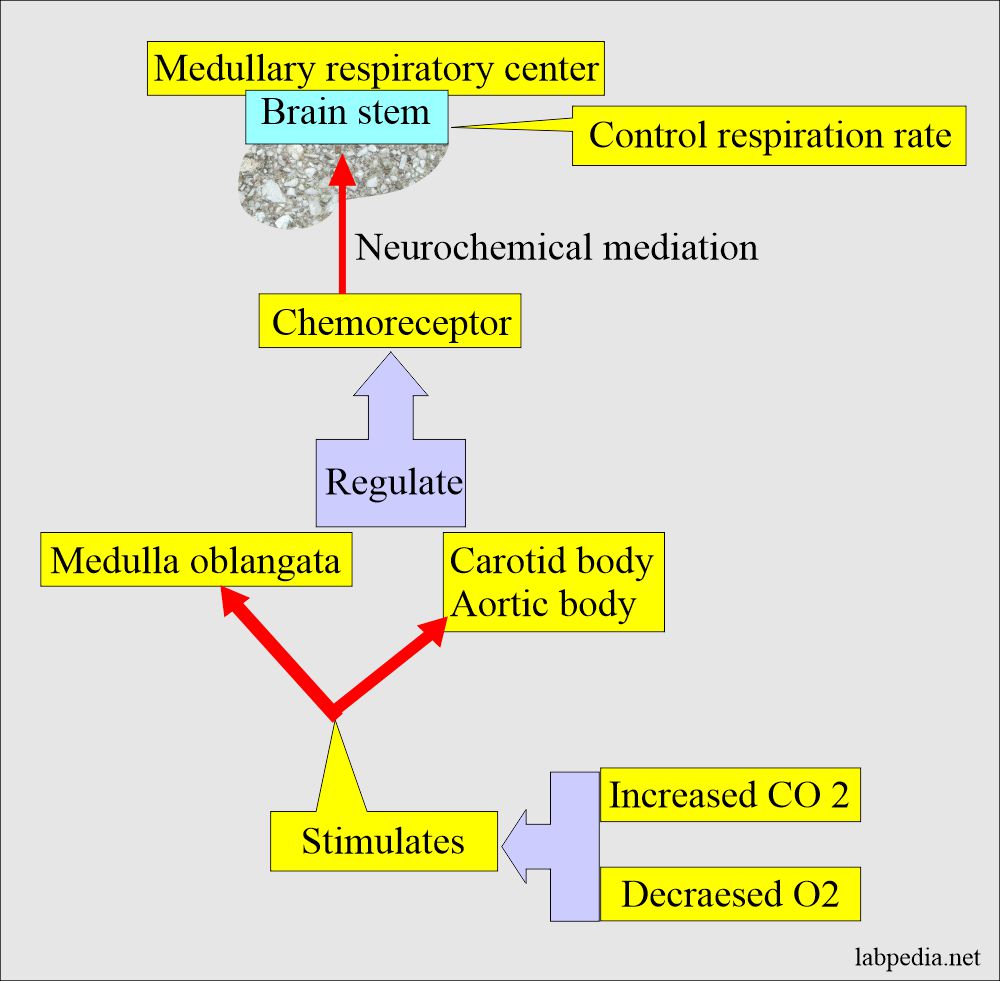
Role of the brain stems in acid-base balance.
Bicarbonate (20 parts)/Carbonic acid (one part):
- = (HCO3 / H2CO3) is the most important buffering system.
- This buffer system works in the lungs as well as in the kidneys.
- The greater the CO2 partial pressure pCO2, the more carbonic acid (H2CO3) forms.
Can express this relationship as :
H2CO3 = 0.03 x pCO2 (mm Hg)
0.03 represents the solubility coefficient for the CO2 in the water.
The pCO2 of the arterial blood is around 44 mm Hg. Therefore the amount of H2CO3 is
equal to 1.2 mmol/L = 0.03 x 40 = 1.2 mmol/L
Acids or chemical substances can donate H+ ions:
- Bases or substances that can accept H+ ions.
- Strong acids readily give up H+, whereas strong base readily accepts H+.
- Respiratory and metabolic disorder depends on the correct measurement of:
- O2
- CO2
- Acid/base assessed by:
- Total CO2
- Plasma pH
- pCO2

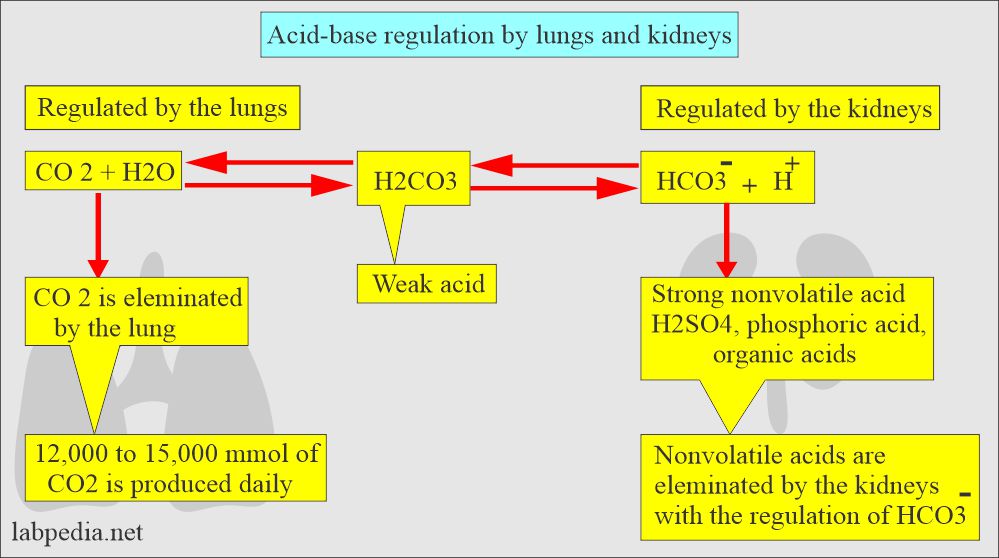
- The lungs can decrease the amount of H2CO3 by blowing off the CO2 and leaving H2O.
H2CO3 → CO2 + H2O - The kidneys can reabsorb HCO3¯or regenerate new HCO3¯ from the CO2 and water.
H2O + CO2 → HCO3¯ + H+
Renal regulation is slow, / Pulmonary regulation is fast.
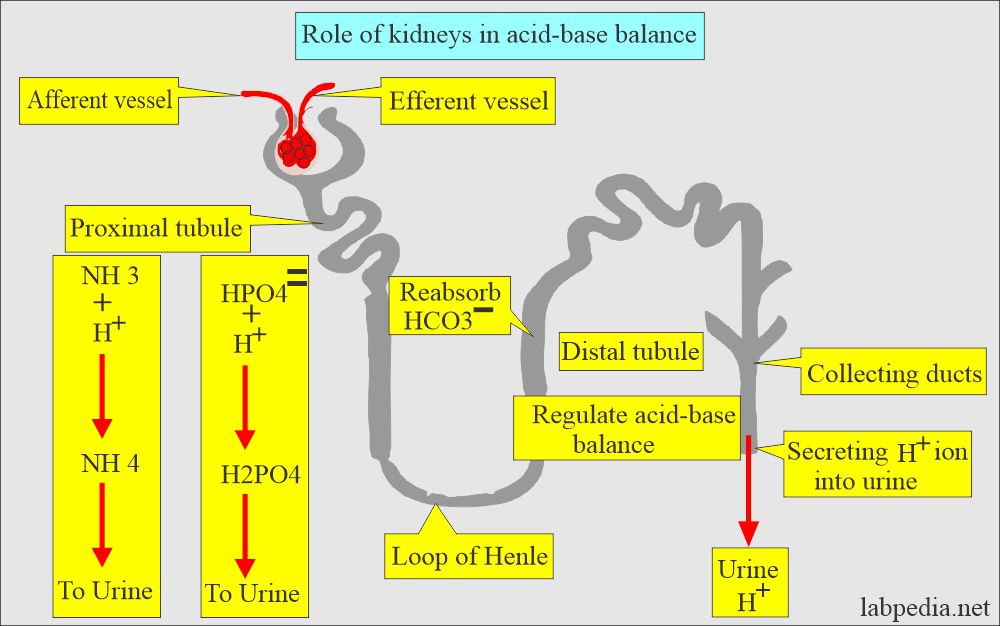
The renal system:
- It works through the excretion or retention of H+, HCO3–, Na+, K+, and Cl¯.
- The distle tubule of the kidney regulates the acid-base balance.
- It secretes H+ ions into the urine and reabsorbs the HCO3¯.
- H2PO4¯ and NH4+ are also secreted into the urine.
The body acids exist in two forms:
- Volatile can be eliminated as CO2.
- Nonvolatile. are sulphuric acid, phosphoric acid, and other organic acids. These are produced by the metabolism of protein, carbohydrates, and fats.
- The volatile acid is carbonic acid (H2CO3) which will form by the hydration of the CO2.
In short, the lungs and kidneys, with the help of buffer systems, are the prime regulator of acid-base balance.
Hydrogen (H+) ions and pH:
- The H+ ions concentration is commonly expressed as the pH; the negative logarithm of H+ ions in the
solution. - The logarithm value means that if the pH changes from 7 to 6, the H+ ions change tenfold.
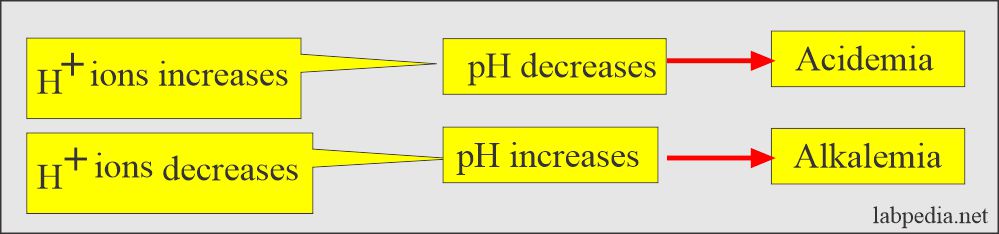
- As the H+ ions increase, the pH decreases; likewise, if the H+ ions decrease, the pH increases.
- The more H+ ions =, the more acidic solution, and the lower the pH.
- The lower the H+ ions =, the more basic solution and higher pH.
- pH <7.4 = acidic.
- pH >7.4 = basic.
| Different body fluids | pH value | Reason for the pH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Henderson- Hasselbalch equation give the idea about the blood gas measurement.
-
- Henderson-Hasselbalch equation = pH = pKa + log (base/acid)
pH calculation formula:
- Where pH = 7.4
- pKa = 6.1
- Base (bicarbonate) = 24
- Acid = dissolved PaCO2 = 0.03 x PaCO2 = 0.03 x 40 = 1.2
- pH = 7.4 = 6.1 + log(24/1.2) = 6.1 + 1.3 = 7.4
- The body makes every effort to maintain the validity of the above equation.
- Our body will maintain a pH of 7.4, and pK is constant to alter the bicarbonate and paCO2
(H2CO3). - The pH is dependent upon the total concentration of:
- CO2.
- HCO–3.
- Dissolved CO2.
- H+ ions.
- All these are interrelated.
- The body normally maintains an arterial pH between 7.35 to 7.45. This takes place through the buffer system of bicarbonate.
| Buffer pairs | Buffer system | pK value | Chemical reaction |
| HCO3¯ / H2CO3 | Bicarbonate/carbonic acid | 6.1 | H+ + HCO3 >< H2O + CO 2 |
| HPO4- /H2PO4- | Phosphate | 6.8 | H2PO4 ↔ H+ + HPO4- |
| Hb / HHb | Hemoglobin | 7.3 | HHb ↔H+ + Hb |
| Pr- /HPr | Protein in blood | 6.7 | HPr ↔ H+ + Pr- |
Common acid/base disorders example is:
- Lactic acidosis and diabetic ketoacidosis.
- Intermediate organic acids are lactic acid and β-hydroxybutyric acid.
- The above acids are metabolized to CO2 and water.
- These may accumulate and cause acidemia.
- Body acids are formed as end products of cellular metabolism. The average person generates acid 50 to 100 meq/day from the metabolism of protein, carbohydrates, fats, and stool loss.
- To neutralize this acid formation, the body needs to maintain the pH in the range; in that case, an equal amount of acid needs to be neutralized or excreted.
Now our various buffer systems come into play to maintain the pH of the body:
- Retention or excretion of H+ ions.
- Respiratory system.
- Renal system.
- These systems are interrelated and work together.