Acid-base Balance:- Part 2 – Metabolic acidosis, Metabolic Alkalosis, and Anion Gap
Metabolic Acidosis, Metabolic Alkalosis
What sample is needed for acid-base balance?
- The better choice is the Radial artery.
- The sample may be taken from the femoral artery or brachial.
- Blood can be drawn from the indwelling arterial line.
- The tests are done immediately because oxygen and carbon dioxide are unstable.
- Place the sample on ice and immediately transfer it to the lab.
- Arterial blood is better than venous blood.
- For a venous blood syringe or tube, be filled, and apply a tourniquet for a few seconds.
- Arterial blood is risky, and a trained person should do it.
- Never apply a tourniquet.
- Don’t apply the pull to the plunger of the syringe.
What are the indications for Acid-base Balance?
It is advised in:
- Diabetes mellitus.
- Starvation.
- Lactic acidosis.
- Ingestion of NH4CL, ethylene glycol, methanol, salicylates, and paraldehyde.
- In the case of diarrhea.
- In the case of renal failure.
- In the case of proximal tubular acidosis.
What are the precautions for the collection of blood?
- Avoid pain and anxiety in the patient, which will lead to hyperventilation.
- Hyperventilation due to any cause leads to decreased CO2 and increased pH.
- Keep blood cool during transit.
- Don’t clench your finger or fist. This will lead to lower CO2 and increased acid metabolites.
- pCO2 values are lower in the sitting or standing position than in the supine position.
- Don’t delay the performance of the test.
- Avoid air bubbles in the syringe.
- Excess of heparin decreases the pCO2 by maybe 40% less.
- Not mixing the blood properly before the test may give a false result.
- A prolonged tourniquet or muscular activity decreases venous pO2 and pH.
- The best way to collect arterial or venous blood is anaerobic.
- Arterial blood precautions:
- Only syringe and needle, no tourniquet, no pull on the plunger.
- Venous blood precautions:
- The needle and syringe of the heparinized evacuated tube were filled and drawn a few seconds after the tourniquet.
- Liquid heparin is the only suitable anticoagulant with the proper amount.
- Less amount will lead to clot formation.
- The increased amount will lead to increased CO2 and a decrease in pH.
- This will lead to a dilutional error.
- Glass collection devices are better than plastic.
How will you define acid-base balance?
- This regulation of the extracellular fluid environment involves the ratio of acid to base, measured clinically as pH.
- Physiologically, all positively charged ions are called acids, and all negatively charged ions are bases.
- Physiological changes in the concentration of H+ ions in the blood lead to acid-base balance.
- A systemic increase in the concentration of H+ ions is called acidosis.
- A systemic decrease in the H+ ions is called alkalosis.
- The acid-base must be regulated within a narrow range for the body to function normally.
- A very slight change in the pH will affect the body.
- A slight change in the H+ ions can change the cell and tissue.
Why H+ ions are needed?
- To maintain the integrity of the membrane.
- Speed of the metabolic reactions.
- Any change in the pH will lead to more harmful effects than other diseases.
- The symbol pH represents the power of H+.
- When pH changes, one unit, like 7.0 to 6.0 = [H+] [H+] = H+ ions, the concentration changes 10-fold.
What is the mechanism of body acid formation?
- Metabolism of proteins.
- Metabolism of Carbohydrates.
- Metabolism of fats.
- This must be balanced by the number of basic substances in the body to maintain the normal pH.
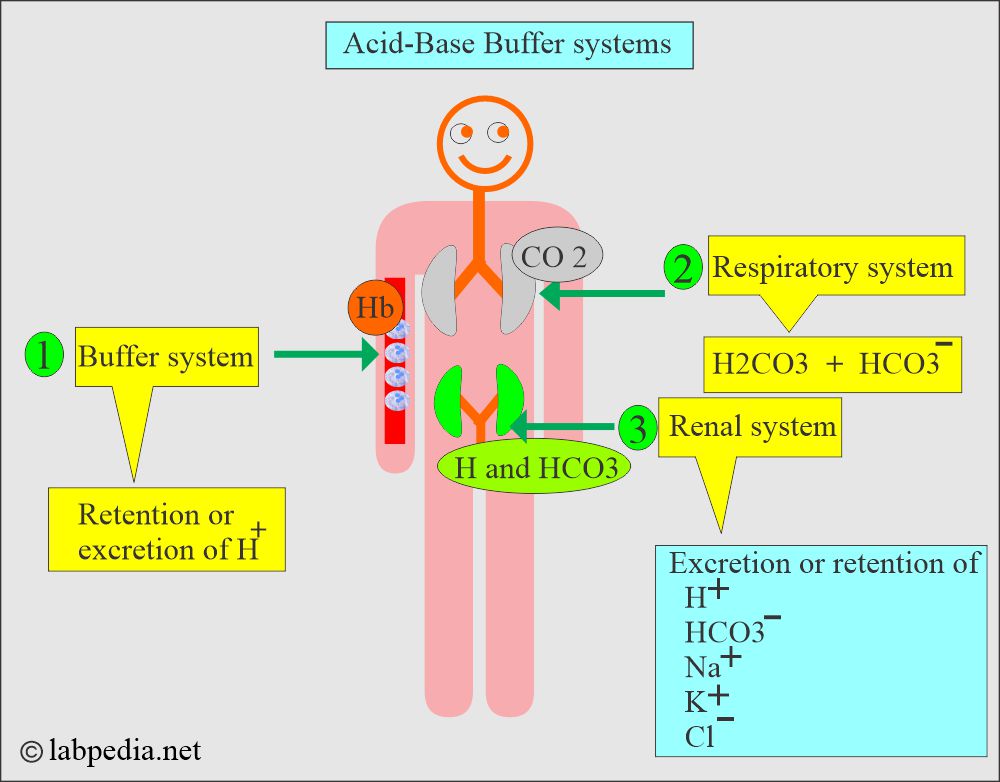
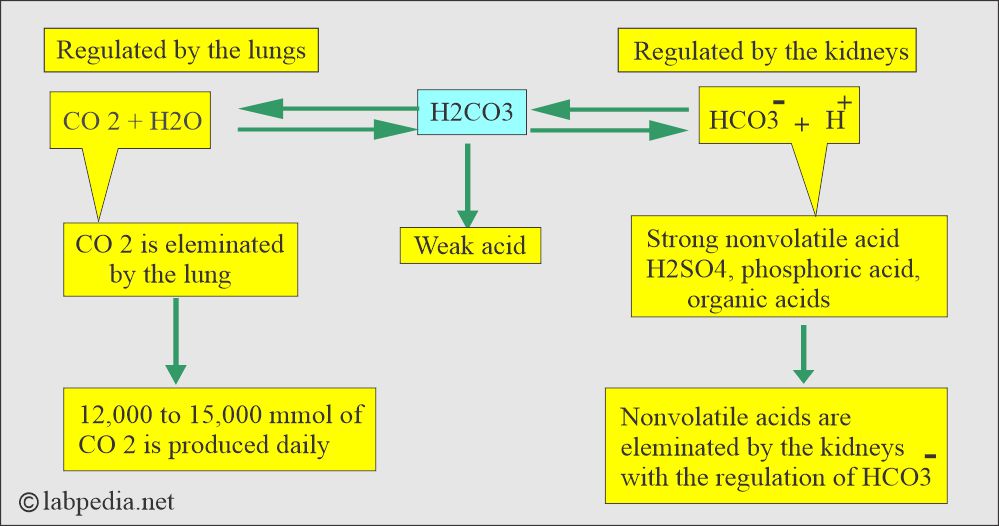
- Lungs, kidneys, and bones are the major organs involved in regulating acid-base balance.
What are the various Buffer Systems?
| Buffer system (pairs) | Buffer system | Buffer reaction | Mechanism |
|
|
|
|
(Carbonic acid/bicarbonate buffer system) |
|
|
|
|
|
– |
|
|
|
|
|
- Acid-base control by the various organs of the body.
What is the pH of different body fluids?
| Body fluids | pH range | Explanation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What is the significance of pH in our life?
- For normal body functions, the pH range is very narrow and needs to be maintained within these limits.
| pH value | Effects on the body |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What are the types of Acid-base balance?
- H+ ions and electrolytes disturbances may be:
- Acute.
- Chronic.
- Modest or severe.
- Simple or mixed.
- When there is an accumulation of H+ ions, it is called acidosis.
- When blood pH declines below 7.3, this process is called acidemia.
- Alkalemia. When there is a deficiency of H+ ions, it is called alkalosis.
- Blood pH rises above 7.45.
- Conditions related to the respiratory system lead to respiratory acidosis or alkalosis.
- There are metabolic conditions related to the kidneys, and abnormal intake/output leads to metabolic acidosis/alkalosis.
What is the importance of blood pH?
- It is normally maintained at 7.38 to 7.42. Any deviation from this range indicates a change in the concentration of H+ ions.
- Blood pH is a negative logarithm of [H+], as shown in the following equation:
- pH = log10 [H+]
- This equation shows that an increase in the H+ ions leads to a fall in the blood pH, which is called acidemia.
- So, a decrease in the H+ ions will lead to an increase in the blood’s pH, which is called alkalemia.
- The conditions that cause the pH change are called acidosis and alkalosis.
- H+ ion changes in the blood lead to acid-base imbalance.
- A systemic increase in the H+ ions is called acidosis.
- In the case of acidemia, the pH of the arterial blood is <7.4.
- In alkalemia, the pH of the arterial blood is >7.4.
- Alkalosis is a systemic decrease in the H+ ions in the systemic blood.
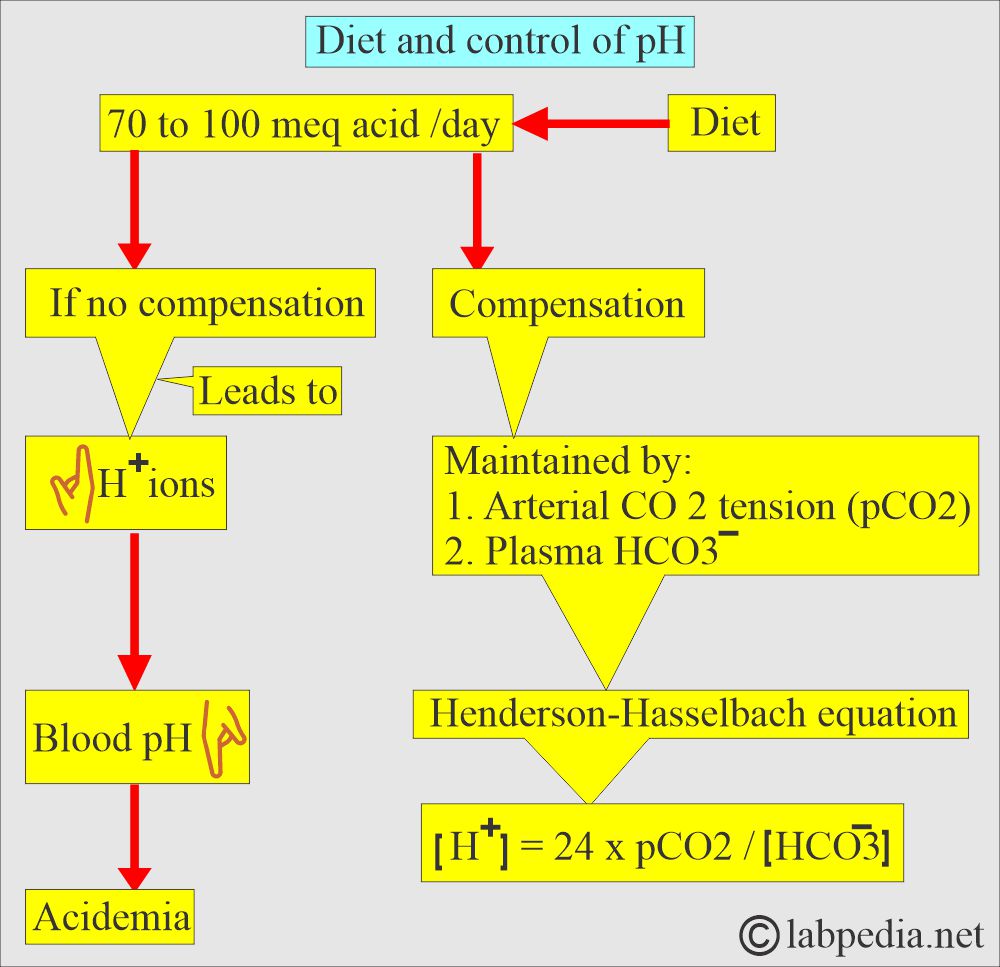
- The following diagram explains how pH is maintained by the arterial carbon dioxide tension (pCO2) and plasma bicarbonate (HCO3–).
- Plasma HCO3– decreases in the plasma caused by gastrointestinal or renal losses will increase H+ ions and lower the pH.
What is the Anion gap?
How will you define the anion gap?
- Anion gap refers to anions usually not measured in the laboratory, like sulfate, phosphate, and lactate.
- The anions usually measured are Chloride (Cl–) and bicarbonate (HCO3–).
- The sum of the anions is subtracted from the sum of cations (Na+ ); there is a gap of around 10 to 12 meq/L called an anion gap. An elevated anion gap gives clues for acidosis.
- The anion gap is measured in meq/L.
- This is the difference between the plasma concentration of the major cation sodium (Na+) and that of the other anions, HCO3—and Cl–.
- Anion gap = [Na+] – ([HCO3–] + [Cl–])
- The normal anion gap is 3 to 13 meq/L, and the mean is 10 meq/L.
- This is dependent mainly on the plasma protein, primarily albumin.
- 2.5 meq/L falls for every 1 gram/dl of albumin concentration in the blood.
- The anion gap is important in identifying the etiology of metabolic acidosis.
What causes a high anion gap (>12 meq/L)?
- Methanol toxicity.
- Uremia due to renal failure.
- Starvation.
- Diabetes mellitus (ketoacidosis).
- Lactic acidosis.
- Salicylates toxicity.
- Ethyl alcohol toxicity.
- Isoniazid toxicity.
- Iron toxicity.
What causes a decreased anion gap (<6 meq/L)?
- Hypoalbuminemia.
- Plasma cell disorders.
- Bromide intoxication.
What causes the normal anion gap (6 to 12 meq/L)?
- Intestinal fistula.
- Pancreatitis.
- Renal tubular acidossis.
- Acid and chloride administration:
- NH4Cl and HCL for the treatment of severe metabolic acidosis.
- Hyperalimentataion.
- Bicarbonate or other alkali losses:
- Diarrhea.
- Recovery from ketoacidosis.
Metabolic acidosis
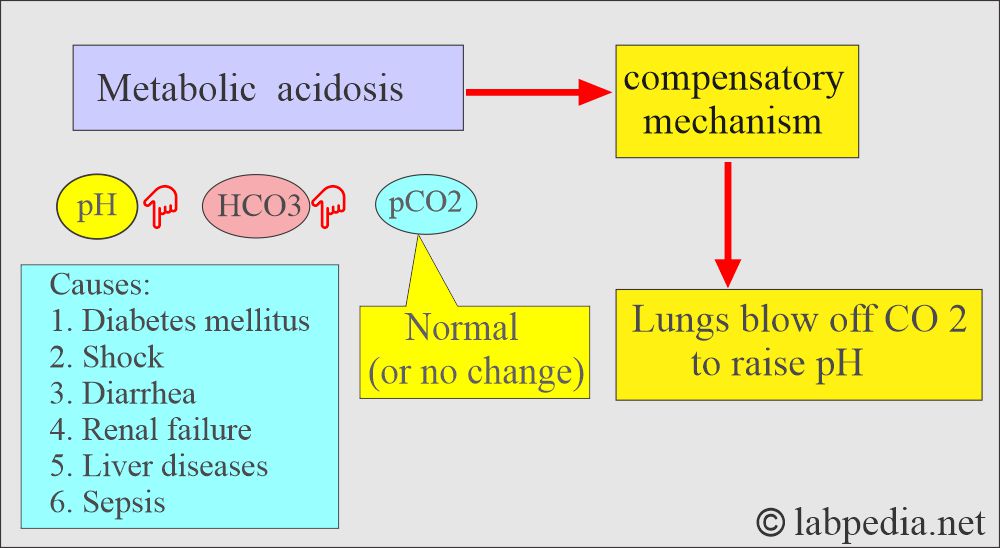
How will you define metabolic acidosis?
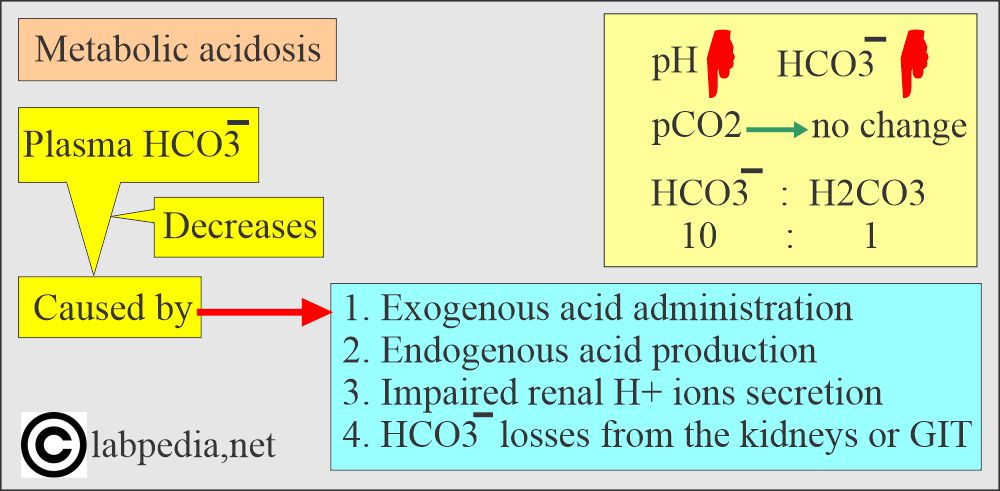
- Metabolic acidosis occurs whenever there is a primary decrease in the HCO3¯ in the blood.
- This may occur due to:
- Exogenous acid administration.
- Endogenous acid production.
- Impaired renal H+ secretion.
- HCO3– losses from the kidney or in the gastrointestinal secretions.
What are the causes of metabolic acidosis?
- In metabolic acidosis, noncarbonic acid increases, or HCO3¯ is lost from the extracellular space.
- The buffering system becomes active and maintains the pH.
- In case of the buffering system’s failure, the anion gap HCO3¯: H2CO3 = 20:1 changes.
- Increased noncarbonic acid with an elevated anion gap and Increased H+ load:
- Diabetes mellitus with ketoacidosis. There is a production of acetoacetic acid and β-hydroxybutyric acid in diabetic acidosis.
- In the case of starvation.
- Lactic acidosis in shock and hypoxemia. There is the production of lactic acid.
- Ingestion of drugs like NH4CL, salicylates, methanol, ethylene glycol, and paraldehyde.
- Decreased H+ ions excretion was seen in:
- Uremia.
- Distal renal tubular acidosis (decreased renal H+ secretion).
- There is an accumulation of the acid that consumes the bicarbonate (HCO3¯).
- Bicarbonate (HCO3¯) loss from the extracellular space and normal anion gap:
- Renal failure.
- Diarrhea.
- Proximal tubular acidosis (there is renal HCO3¯ loss).
- Plasma HCO3¯ falls, associated with a rise in the concentration of the inorganic anions, mostly CL¯ or a fall in Na+ concentration.
What are the biochemical changes in metabolic acidosis?
| Biochemical parameters | Value |
|
|
|
|
|
|
|
|
How is there compensation for metabolic acidosis?
- Hyperventilation by rapid breathing from the lungs will blow off CO2.
- Kidneys will conserve HCO3¯ and eliminate H+ ions in the urine, where urine will be acidic.
What are the signs and symptoms of metabolic acidosis?
- Kussmaul respiration suggests metabolic acidosis.
- The early symptoms are headache and lethargy.
- There is anorexia, nausea, vomiting, diarrhea, and abdominal discomfort.
- If acidosis progresses, then ultimately, the end is death.
- The patient can have neurological, respiratory, gastrointestinal, and cardiovascular signs and symptoms.
- Deep rapid respiration indicates respiratory compensation.
- There is increased tidal volume rather than respiratory rate, which characterizes these ventilatory changes resulting from the low pH stimulating the brain stem respiratory center.
- Decreased blood pH leads to:
- Decreased myocardial contraction, causing decreased blood pressure.
- Arterial vasodilatation.
- pH below 7.15 to 7.20, the effect of acidemia is prominent.
- Ketoacidosis is associated with increased thirst and polyuria.
- There is secondary hypotension in severe acidotic patients.
- Severe acidosis produces life-threatening dysrhythmias, like ventricular fibrillation.
- Ultimately, the patient will go into a coma.
How will you diagnose metabolic acidosis?
- Take the history of the patient.
- There are clinical signs and symptoms.
- Lab. findings are:
- pH = <7.35. (low pH).
- HCO3¯ = <24 meq/L (low plasma bicarbonate).
- Anion gap = >14 meq/L seen in:
- High-anion metabolic acidosis.
- Lactic acidosis.
- Ketoacidosis.
- Asprin over-dose.
- Renal failure.
- Overuse of alcohol.
- Anion gap = 12 meq/L or less is seen in:
- Increased acid load.
- Rapid I/V saline administration.
- Other diseases are characterized by HCO3– loss.
- Urine pH = <4.5 in the absence of renal disease.
- Lactic acid = Increased (in lactic acidosis).
- There may be concomitant hypokalemia or hyperkalemia, which helps in the diagnosis.
How will you treat metabolic acidosis?
- Until arterial pH falls below 7.15 to 7.20, acidemia’s adverse effect is usually compensated for by elevated plasma catecholamines.
- In case of severe acidosis, give NaHCO3 to elevate the pH.
- Correct the sodium and water deficiency.
- Give lactate ringer solution.
- Correct the electrolyte imbalance.
- Try to treat the underlying cause of the acidosis.
- In case mechanical ventilation may be needed.
- May need dialysis for patients with renal failure.
- Needs antibiotics to treat the infection.
Metabolic Alkalosis
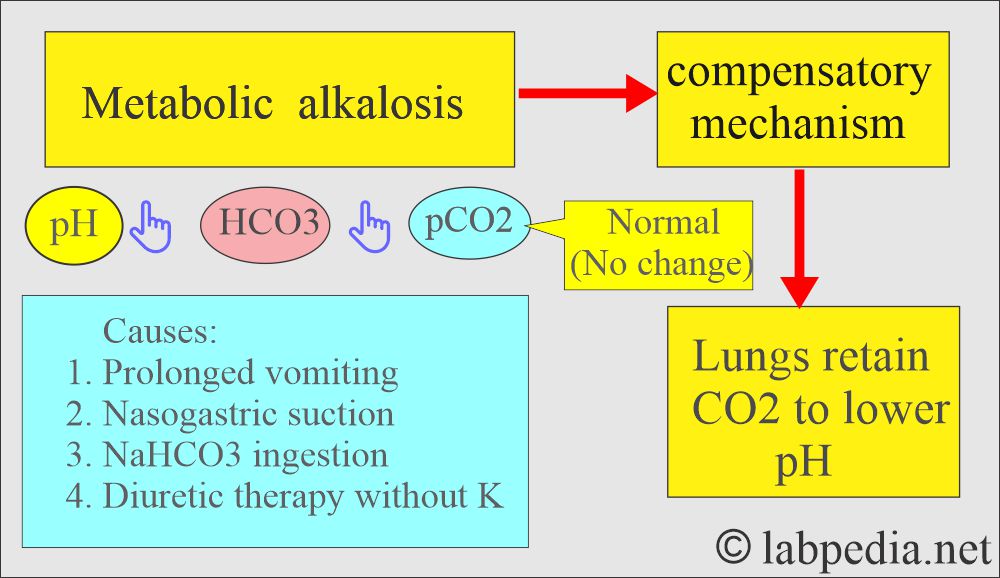
How will you define metabolic alkalosis?
- There is excessive loss of metabolic acids.
- An increase in the plasma HCO3‾.
- An arterial pH >7.4 leads to metabolic alkalosis.
What are the causes of metabolic alkalosis?
- This common condition is often induced by diuretic therapy or loss of gastric secretions (in vomiting or nasogastric suction).
- This condition is caused by:
- There must be an initial increase in the HCO3— level caused by the loss of H+ ions in the gastrointestinal secretions or the urine.
- H+ ions move into the cell.
- Akali administration.
- Volume contraction around a relatively constant amount of extracellular HCO3-.
- One of the following factors in case of absence of renal failure to maintain high HCO3–:
- Chloride (Cl–) depletion.
- Hypochloremia or hypokalemia.
- Effective circulating volume depletion.
- This occurs in the acid loss by vomiting or nasogastric suction.
- Pyloric or upper duodenal obstruction.
- In the case of villous adenoma.
- Prolonged diuretic therapy.
- Cystic fibrosis.
- Primary Hyperaldosteronism leads to retention of the NaHCO3 and loss of H+ and K+.
- Secondary hyperaldosteronism.
- Bilateral adrenal hyperplasia.
- Congenital adrenal hyperplasia.
- Cushing’s syndrome.
- Pituitary adenoma secreting ACTH (Cushing’s syndrome).
- Exogenous cortical therapy.
- Excessive licorice ingestion.
- Diuretics also produce mild alkalosis because they produce more Na+, K+, and CL¯ excretion than HCO3¯.
- Milk-alkali syndrome.
- Massive blood transfusion.
- High doses of carbenicillin or penicillin.
What are the changes in metabolic alkalosis?
| Biochemical parameters | Value |
|
|
|
|
|
|
How will you summarize metabolic alkalosis?
- Before alkalosis, the HCO3: H2CO3 ratio was 20:1.
- Then pH increases, PCO2 no change, and HCO3¯ also increases.
- HCO3: H2CO3 = 40 :1
- HCO3¯ increases because of the loss of CL¯ ions or excess ingestion of NaHCO3.
What is the compensatory mechanism of metabolic alkalosis?
- Breathing will be suppressed to hold the CO2.
- The increase in the pH depresses the respiratory center, causing CO2 to be retained, which will increase H2CO3 and CO2.
- The kidney will conserve H+ ions and excrete more HCO3¯ in the urine, which will be alkaline.
What are the signs and symptoms of metabolic alkalosis?
- The patients are irritated, twitching, and confused.
- There are nausea, vomiting, and diarrhea.
- Some patients may have severe cramping, paresthesia, or even tetany, but in others with similar electrolytes, data have no such S/S; the reason is unknown.
- Ask about the history of vomiting or diuretic therapy.
- There is a weakness.
- There are muscle cramps.
- There are hyperactive reflexes.
- There is shallow and slow respiration. There are cyanosis and apnoea.
- There will be tetany.
- The patient will have confusion and convulsions.
- There are cardiovascular abnormalities due to hypokalemia.
- Ultimately, the patient will have atrial tachycardia.
How will you diagnose metabolic alkalosis?
- The arterial blood shows increased pH and HCO3¯.
- pH = >7.45.
- HCO3– = >29 meq/L.
- K+ = <3.5 meq/L (low).
- Calcium (Ca++)= <8.9 mg/dl.
- Chloride (Cl–) = <98 meq/L.
- There may be an increased anion gap.
- Measurement of the Na+ in a random urine sample differentiates urinary volume depletion Na+ <20 meq/L and euvolemic Na+ >40 meq/L.
- Metabolic alkalosis is the condition in which volume depletion may not lead to a low urinary Na+.
- The capacity to retain the Na+ in this situation may be antagonized by the need to excrete HCO3– (as Na+ salt) to correct the alkalosis.
- In such cases, a random urinary Cl– determination is more useful.
How will you summarize metabolic alkalosis?
| Lab parameter | Value |
|
|
|
|
|
|
|
|
|
|
|
|
How will you treat metabolic alkalosis?
- In the case of mild alkalosis, the patient can tolerate it.
- In the case of severe cases of pH >7.6, urgent treatment is needed.
- Can give KCl and normal saline.
- Discontinue diuretics and supplementary KCl.
How will you summarize the characteristic features of acidosis and alkalosis?
| Clinical condition | Etiology of the condition | pH (7.37 to 7.43) | HCO3– (19 to 25 meq/L) | pCO2 (38 to 42 mmHg) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What are the Panic values?
| Clinical parameter | Panic value |
|
|
|
|
|
|
What are the parameters needed for the acid-base balance?
| Lab test | Importance |
|
|
|
|
|
|
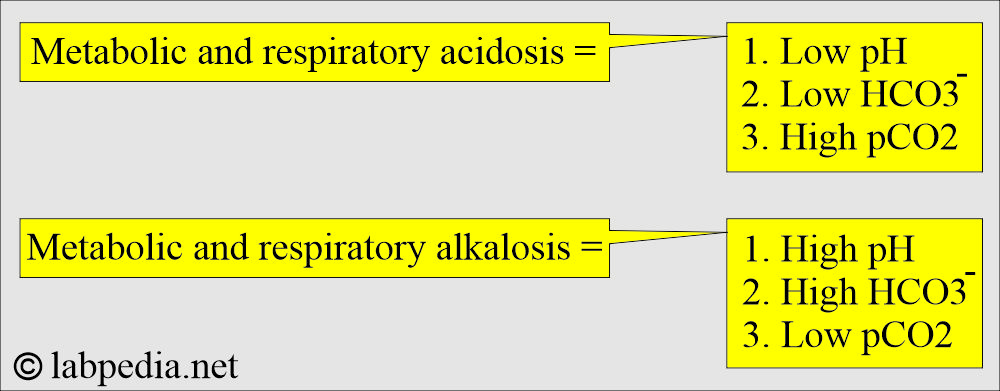
How will you summarize metabolic and respiratory acidosis/alkalosis?
| Clinical condition | pH | HCO3– | pCO2 |
|
|||
| Acute metabolic acidosis | Decreased | Decreased | Normal |
| Compensated metabolic acidosis | Normal | Decreased | Decreased |
| Acute respiratory acidosiss | Decreased | Normal | Increased |
| Acute compensated respiratory acidosis | Normal | Increased | Increased |
|
|||
| Acute metabolic alkalosis | Increased | Increased | Normal |
| Chronic metabolic alkalosis | Increased | Increased | Increased |
| Acute respiratory alkalosis | Increased | Normal | Decreased |
| Compensated respiratory alkalosis | Normal | Decreased | Decreased |
Questions and answers:
Question 1: What is the panic value in acid-base balance?
Question 2: What are decreased anion gap causes.

Thank you
Thanks.