Acid-base Balance:- Part 5 – Bicarbonate Level (HCO3-)
Bicarbonate Level (HCO3–)
What sample is needed for Bicarbonate Level (HCO3–)?
- It is done on the patient’s serum or plasma (plasma arterial or venous can be used).
- The best anticoagulant is heparin.
- The test should be done as soon as possible, and the time interval between taking and analyzing a sample should be minimized.
- Collect samples anaerobically, and heparin is the preferred anticoagulant.
What are the indications for Bicarbonate Level (HCO3–)?
- It assists in evaluating the pH of the patient.
- It also assists in evaluating electrolyte balance.
- The bicarbonate ion measures a metabolic (renal) component of the acid-base equilibrium.
How will you define Bicarbonate Level (HCO3–)?
- This is the second most plasma anion after chloride.
- As an index bicarbonate ions concentration, this measures the total CO2 in the blood (serum).
- >90% of blood CO2 exists in the ionized HCO3– form, which is converted to CO2 by adding a standard amount of acid in the serum.
- Arterial blood has less CO2 than venous blood.
- For the result’s uniformity, total CO2 is done on venous blood serum where the normal range is 19 to 25 meq/L.
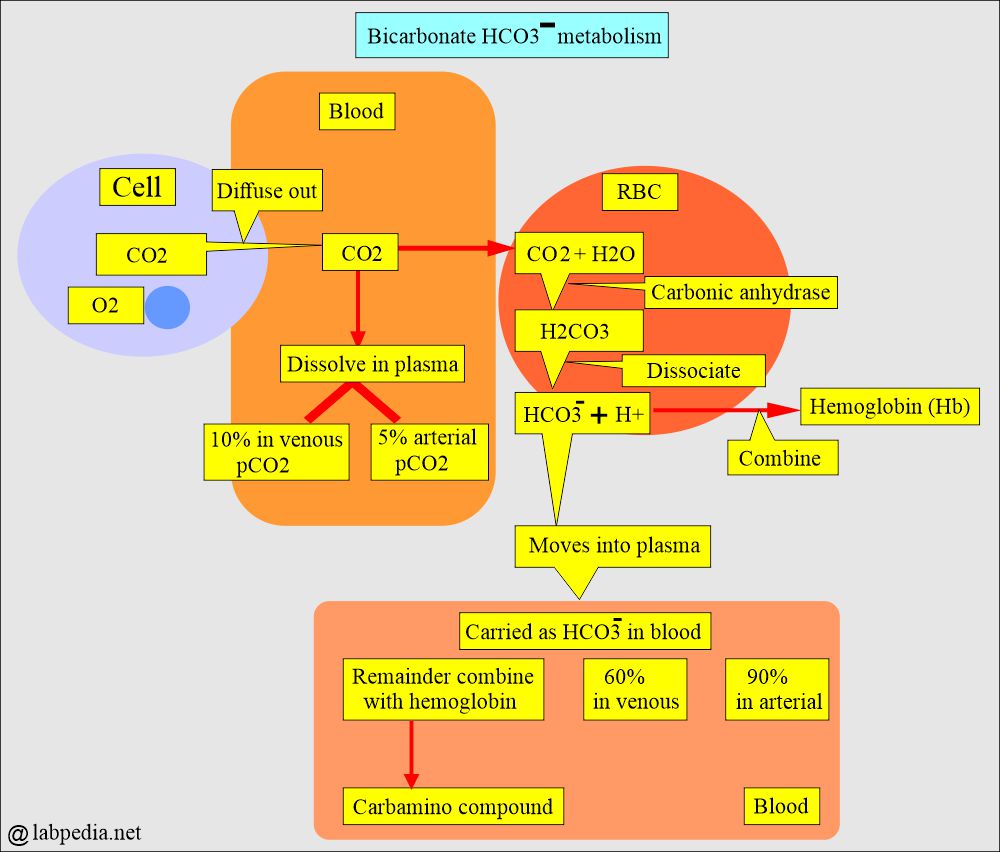
- The CO2 contents measure H2CO3, dissolved CO2, and the HCO3– anions.
- H2CO3 and dissolved CO2 contents in the blood are so small that CO2 contents are an indirect measure of HCO3– anions.
- HCO3– Play a major role in the acid-base balance.
How will you discuss the pathophysiology of Bicarbonate Level (HCO3–)?
- Bicarbonate is the most important buffer system in the blood, maintaining the pH (acid-base balance).
- H+ + HCO3 –↔ H2O + CO2
- Buffer pair = HCO3– / H2CO3
- The ratio = HCO3– / H2CO3 = 20:1
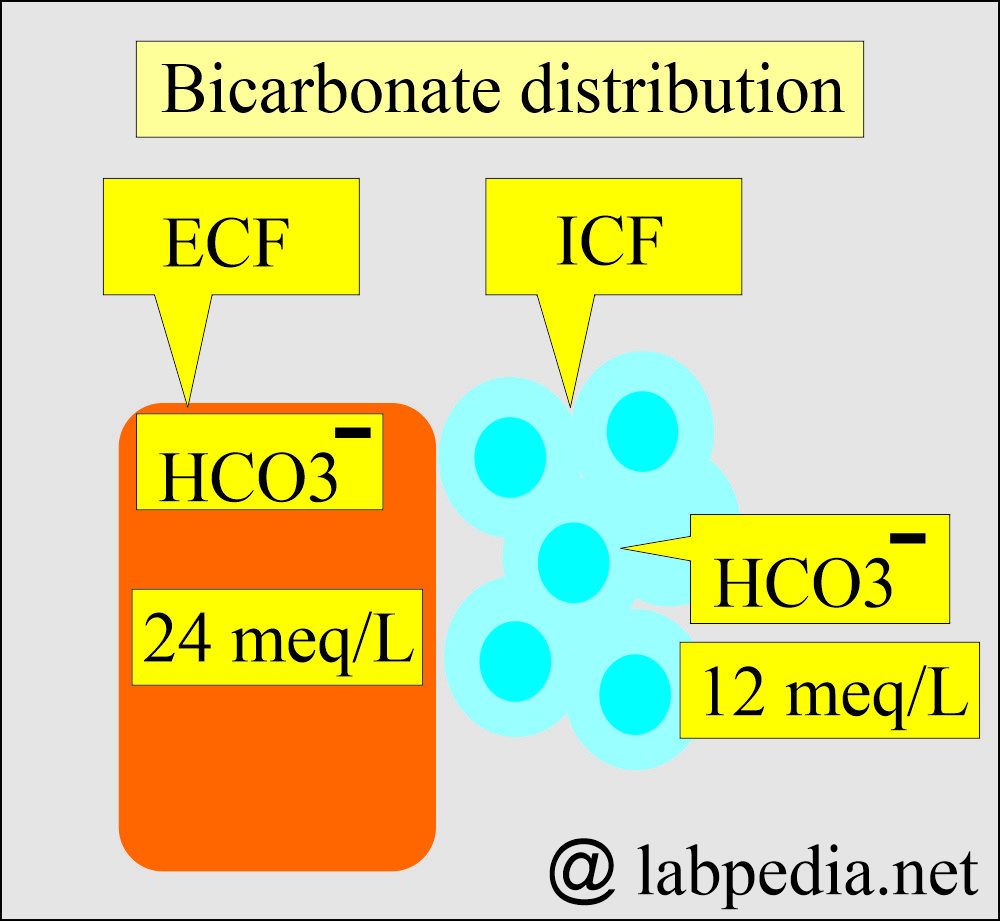
- HCO3– = 24 meq/L (ECF)
- HCO3– = 12 meq/L (ICF)
- Carbonic acid = 1.2 meq/L
- Normal pH = 7.4
- Correction occurs when the values for both components of the buffer pair (HCO3 / H2CO3) return to normal.
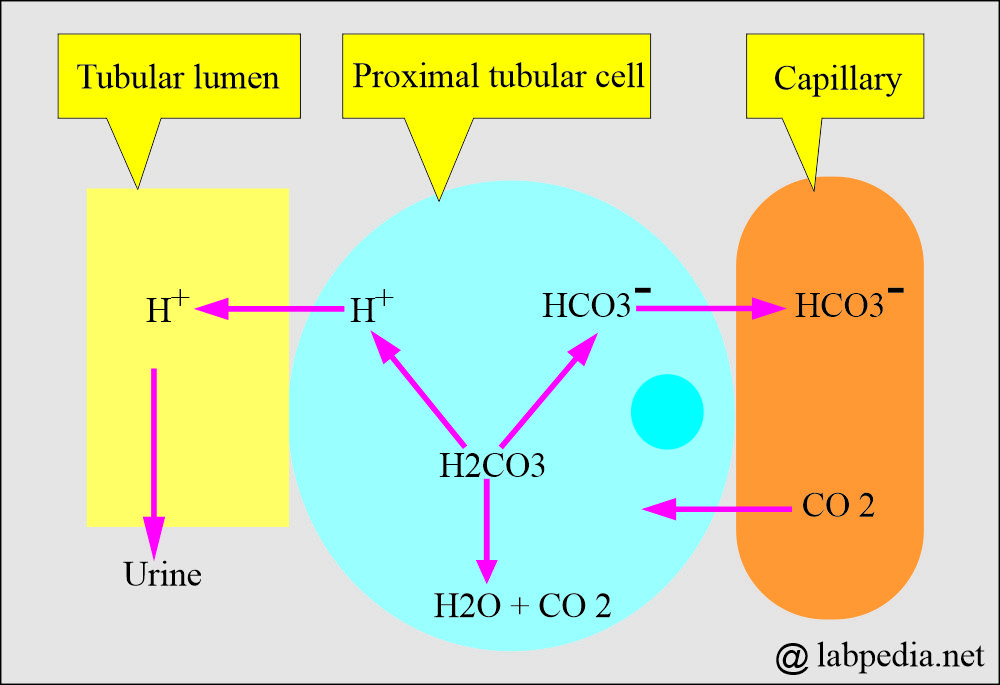
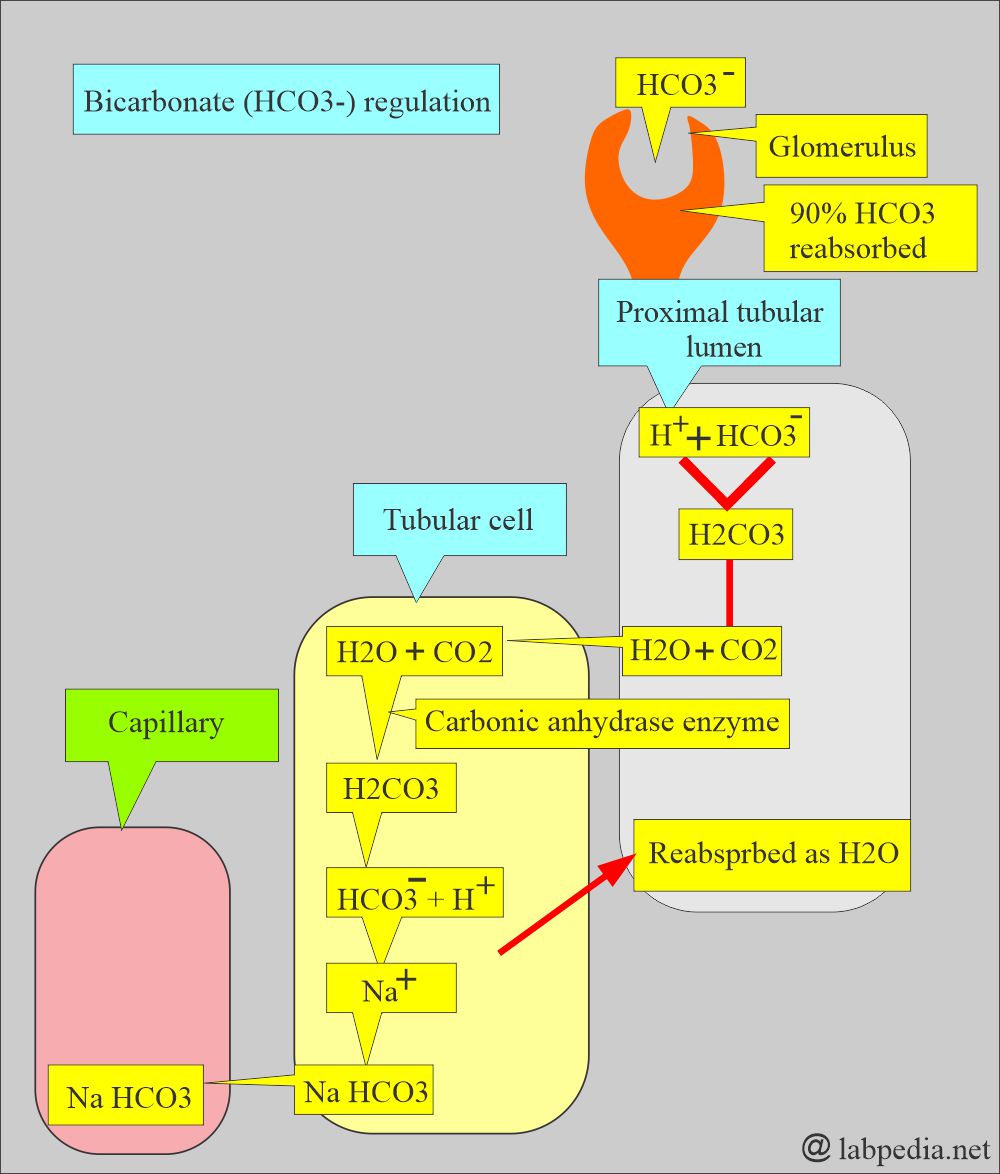
- Bicarbonate measures a metabolic (Kidney) component of acid-base balance.
- The kidney easily regulates bicarbonate, which excretes excess and retains it when needed.
- This buffer pair (HCO3–/H2CO3) operates in the kidneys and the lungs and is the major extracellular buffer.
- Most of the CO2 in the body is in the form of HCO3-, so the CO2 level in the blood measures HCO3–.
- The CO2 contents measure H2CO3, dissolved CO2, and the bicarbonate (HCO3–) ions present in the blood.
How will CO2 be carried in the blood?
- Dissolved in the plasma (pCO2).
- As bicarbonate (HCO3–).
- Carbamino compound.
- Various respiratory and metabolic disturbances affect the bicarbonate level, affecting acid-base balance.
- HCO3– ion measures the metabolic kidney part of the acid-base balance.
- HCO3– is exchanged for other ions like Chloride and Phosphate to maintain electroneutrality.
- When the HCO3 level increases, the pH also increases.
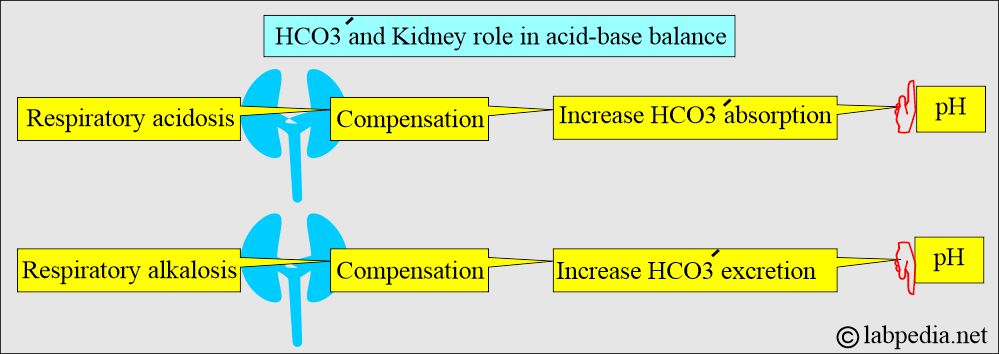
What is the role of the Kidney in acid-base balance?
- Kidneys play an important role in the balance of the acid-base system (compensation).
- Kidneys compensate by producing more acidic or alkaline urine.
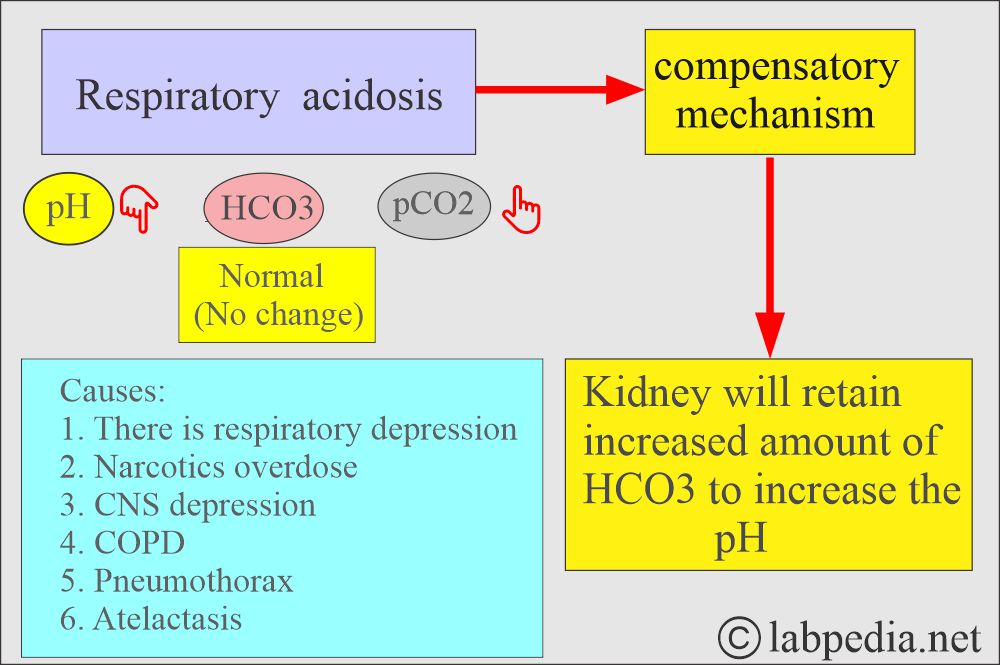
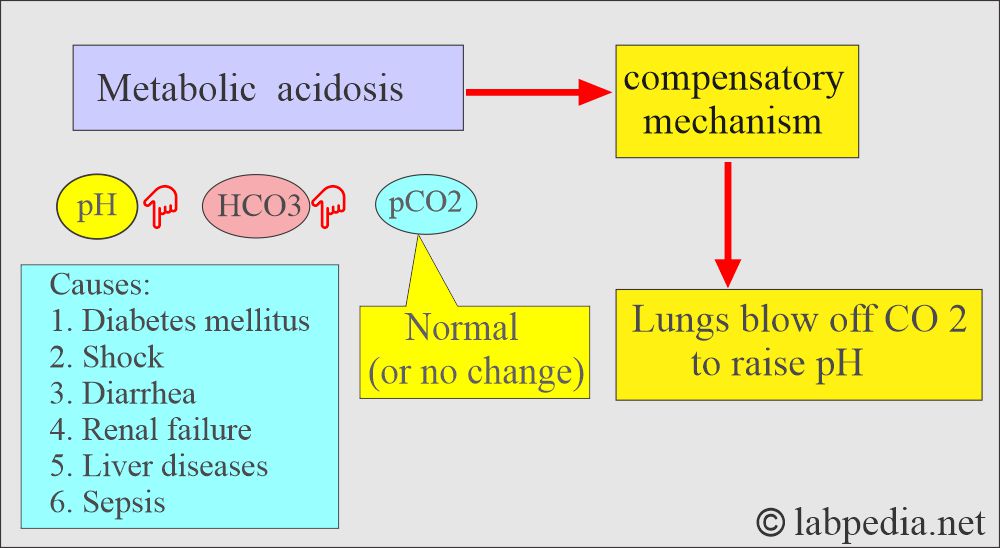
- In respiratory acidosis, the kidney compensates by increased reabsorption of HCO3–.
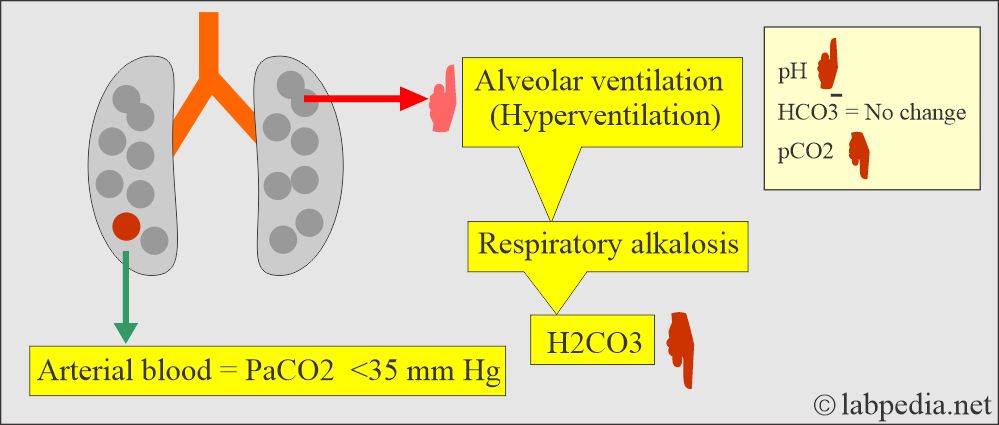
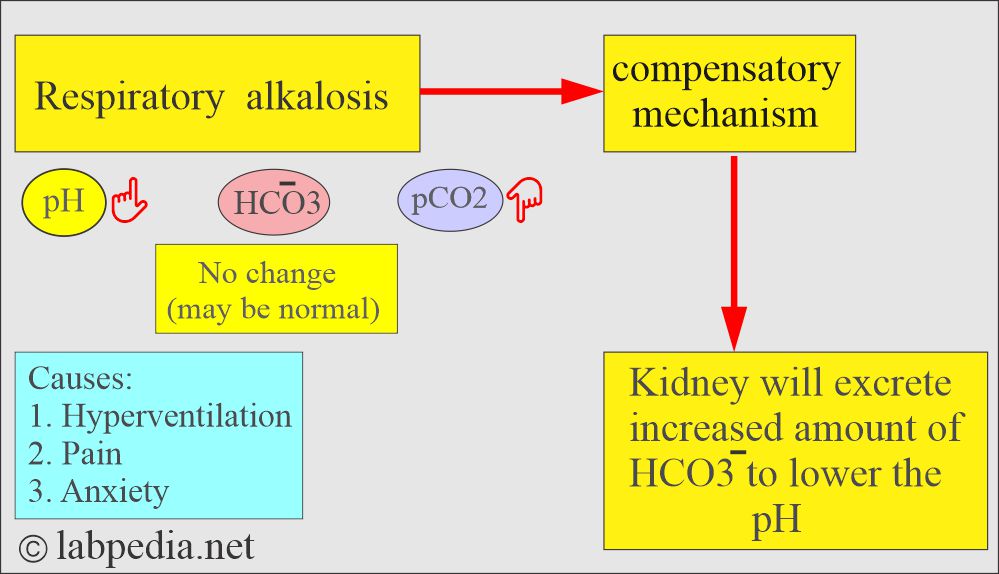
- In respiratory alkalosis, the kidney compensates by increased excretion of HCO3–.
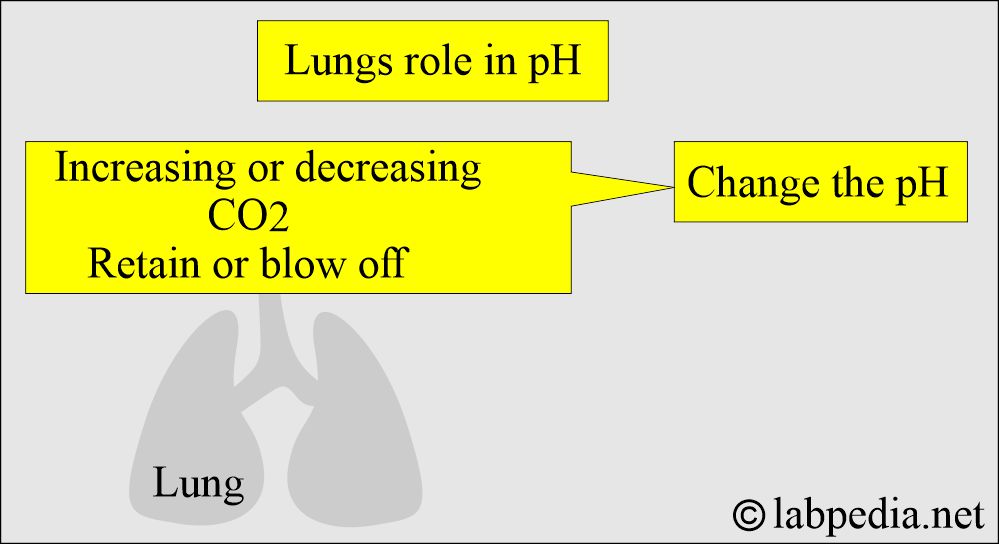
What is the role of Lungs in acid-base balance?
- Lungs compensate by increased or decreased blow-off of CO2, and this will change the pH.
What is the normal Bicarbonate Level (HCO3–)?
- Arterial blood = 21 to 28 meq/L
- Venous blood = 22 to 29 meq/L
- Peritoneal fluid = 24 to 29 meq/L
- Duodenal fluid = 4 to 21 meq/L
- Pancreatic fluid = 66 to 127 meq/L
- For SI, the unit multiplication factor is 1, which will be in mmol/L
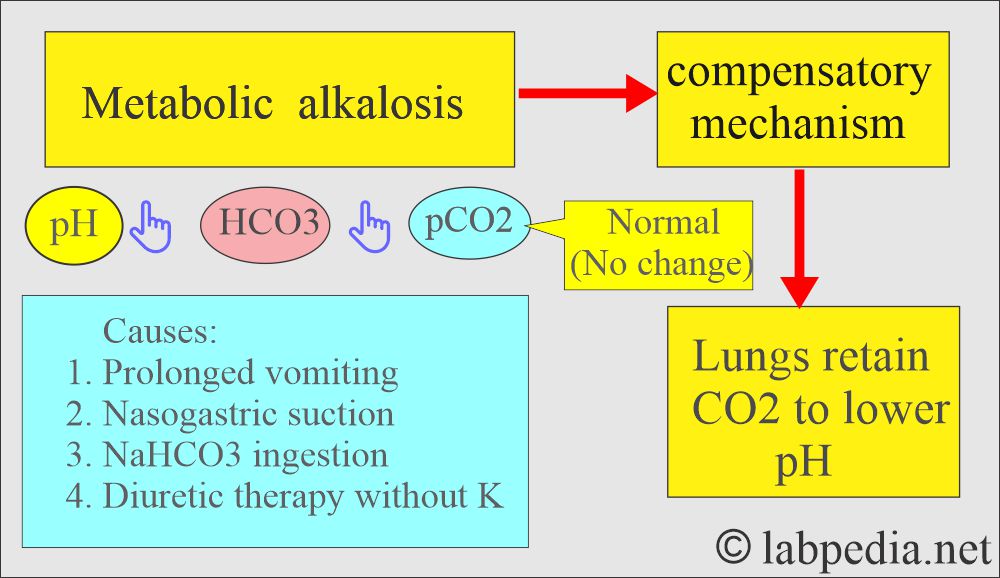
What are the causes of decreased Bicarbonate (HCO3-) levels?
- Addison disease
- Diarrhea
- Ethylene glycol poisoning
- Ketoacidosis
- Kidney disease
- Lactic acidosis
- Metabolic acidosis
- Starvation.
- diabetic ketoacidosis.
- Methanol poisoning
- Salicylate toxicity (such as aspirin overdose)
- Liver disease
What are the causes of increased Bicarbonate (HCO3-) levels?
- Breathing disorders (compensated respiratory acidosis)
- Cushing syndrome
- Excessive vomiting
- Hyperaldosteronism
- Ingestion of excessive amounts of antacids, diuretics, and steroids
- Severe vomiting.
What are the conditions that may alter bicarbonate (HCO3–) levels?
- Alkalosis
- Delirium
- Dementia
- Renal tubular acidosis, distal.
- Renal tubular acidosis, proximal.
Acid-base balance and HCO3– level
- Acidemia means arterial blood pH <7.4.
- Acidosis means a systemic increase in H+ ions.
- Alkalemia means arterial blood pH >7.4.
- Alkalosis means a systemic decrease in H+ ions.
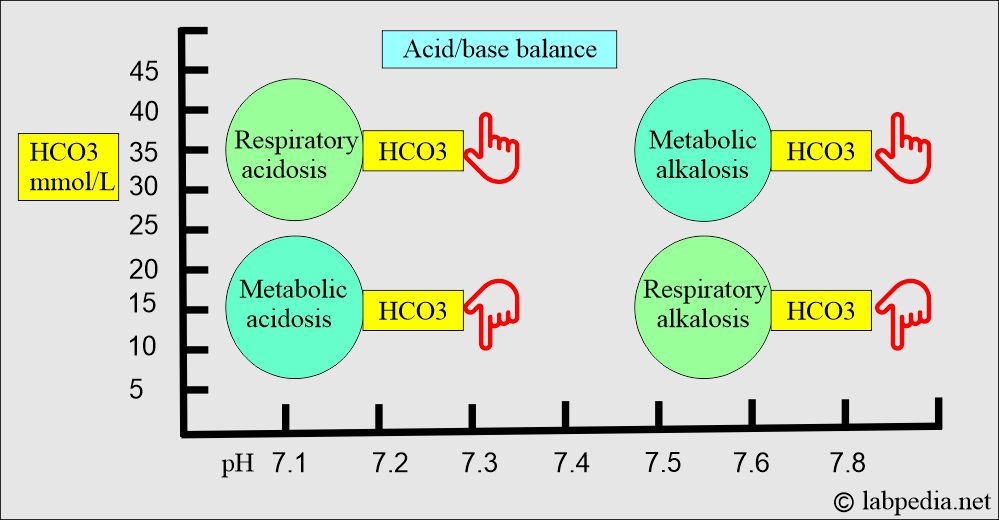
Respiratory acidosis:
- An absolute CO2 excess results in decreased pH, increased pCO2, and a base deficit.
Respiratory alkalosis:
- There is an absolute CO2 deficit that results in increased pH and decreases pCO2 and base excess.
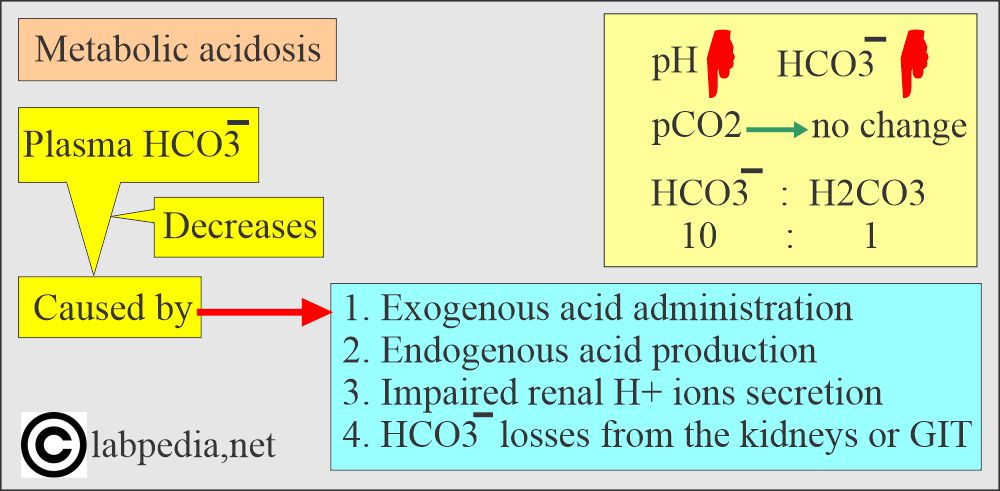
Metabolic acidosis:
- There is an absolute HCO3 deficit, resulting in decreased pH and HCO3.
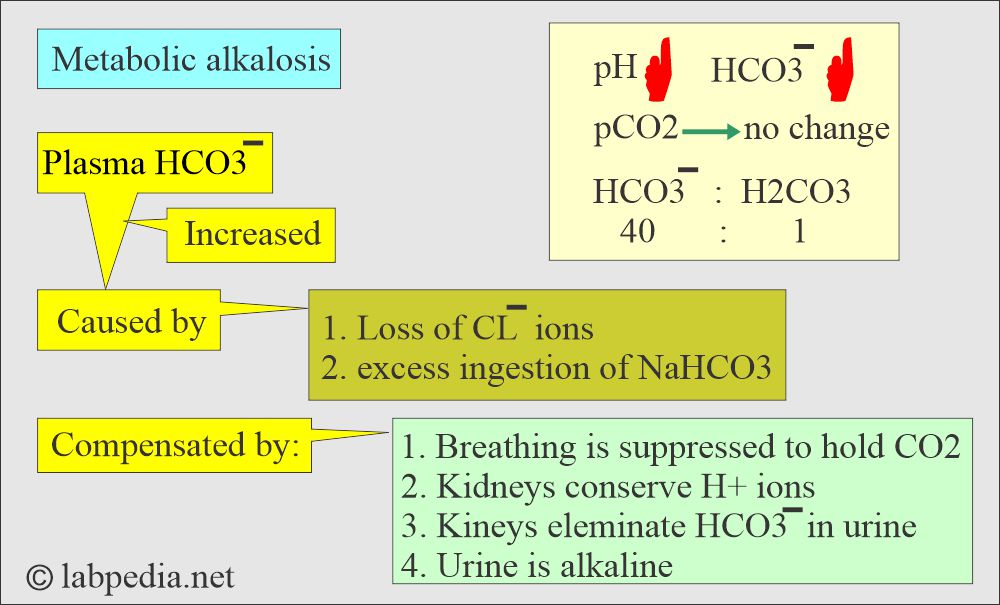
Metabolic alkalosis:
- There is absolute HCO3 excess, resulting in increased pH and HCO3 levels.
What are the Acid-base balance values in different conditions?
| HCO3 | pCO2 | pH | Etiology | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
What are the Panic values?
| Clinical parameter | Panic value |
|
|
|
|
|
|
What parameters are needed for acid-base balance?
| Lab test | Importance |
|
This will tell:
|
|
This is the partial pressure of CO2, and it will tell:
|
|
This is the partial pressure of the O2 in the arterial blood and tells:
|
Questions and answers:
Question 1: What is the panic value of pH?
Question 2: What is the role of lungs in acid-base balance?

Very nice ,precise,understandable and applicable content
The topic is upgraded. Thanks for your remarks.