Chapter 27: Autoimmune diseases: Autoimmune Hemolytic Anemia (AHA)
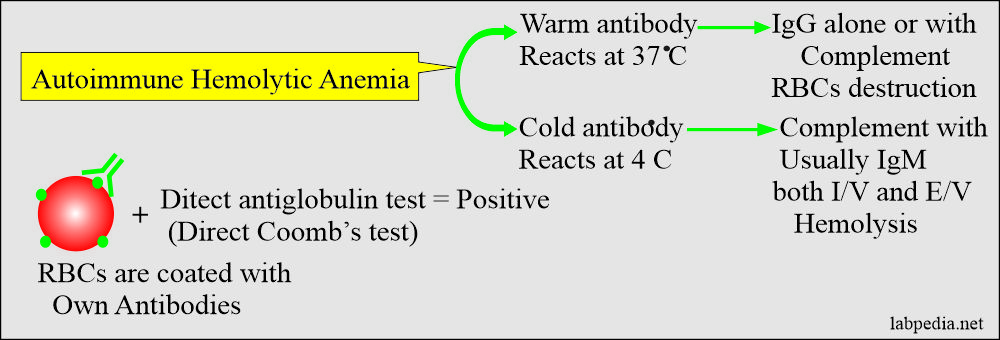
AUTOIMMUNE HEMOLYTIC ANAEMIA (AHA)
Definition of autoimmune hemolytic anemia
AHA represents the abnormality in the immune system where self-recognition of the RBC antigens is lost.
In autoimmune hemolytic anemia (AHA), patients produce antibodies to their own RBCs. The direct Coomb’s test is positive, which indicates that RBCs are coated with autoantibodies.
Differential diagnosis of the hemolytic anemia in comparison to intravascular or extravascular hemolysis:
| Laboratory parameters | Intravascular hemolysis | Extravascular hemolysis |
| Hemoglobin | Decreased | Decreased |
| Hematocrit (Hct) | Decreased | Decreased |
| Red blood cells morphology | Depend upon the type of antibody | Phagocytosed by the MN photosystem, more spherocytes |
| Reticulocytes | >1% (increased) | >1% (increased) |
| Hemoglobinuria | Positive | Absent |
| Hemoglobinemia | Positive | Absent |
| Serum bilirubin total and indirect | Increased | Increased |
| Lactate dehydrogenase (isoenzymes LD1) | Increased | Increased |
| Haptoglobin | Decreased | Decreased |
Autoimmune Haemolytic Anaemia may be:
- Primary.
- Secondary to other causes like malignancy or inflammatory diseases.
Autoimmune Hemolytic Anaemias are classified into:
- Warm reactive autoantibody, which reacts at 37 oC. This is the most common autoimmune hemolytic anemia.
- Idiopathic warm AHA.
- Secondary types of AHA is due to:
- Connective tissue diseases like SLE, Rheumatoid arthritis.
- Autoimmune hepatitis.
- Lymphoproliferative disorders like lymphocytic leukemia, Non-Hodgkin’s lymphoma.
- Viral infections like Hepatitis B-virus.
- A cold reactive autoantibody called cold autoimmune H.A, which reacts below 37Co.
- The optimal activity is at 4 °C but can react between 25 °C to 31 °C.
- This is seen in <20% of the cases.
- Drugs induced hemolysis is seen in <20% of the cases.
- This is described in two forms:
- Cold agglutinin syndrome.
- Paroxysmal cold hemoglobinuria.
- Drug-induced autoimmune hemolytic anemia, representing in some of the studies around 12%.
- Cold paroxysmal hemoglobinuria is rare.
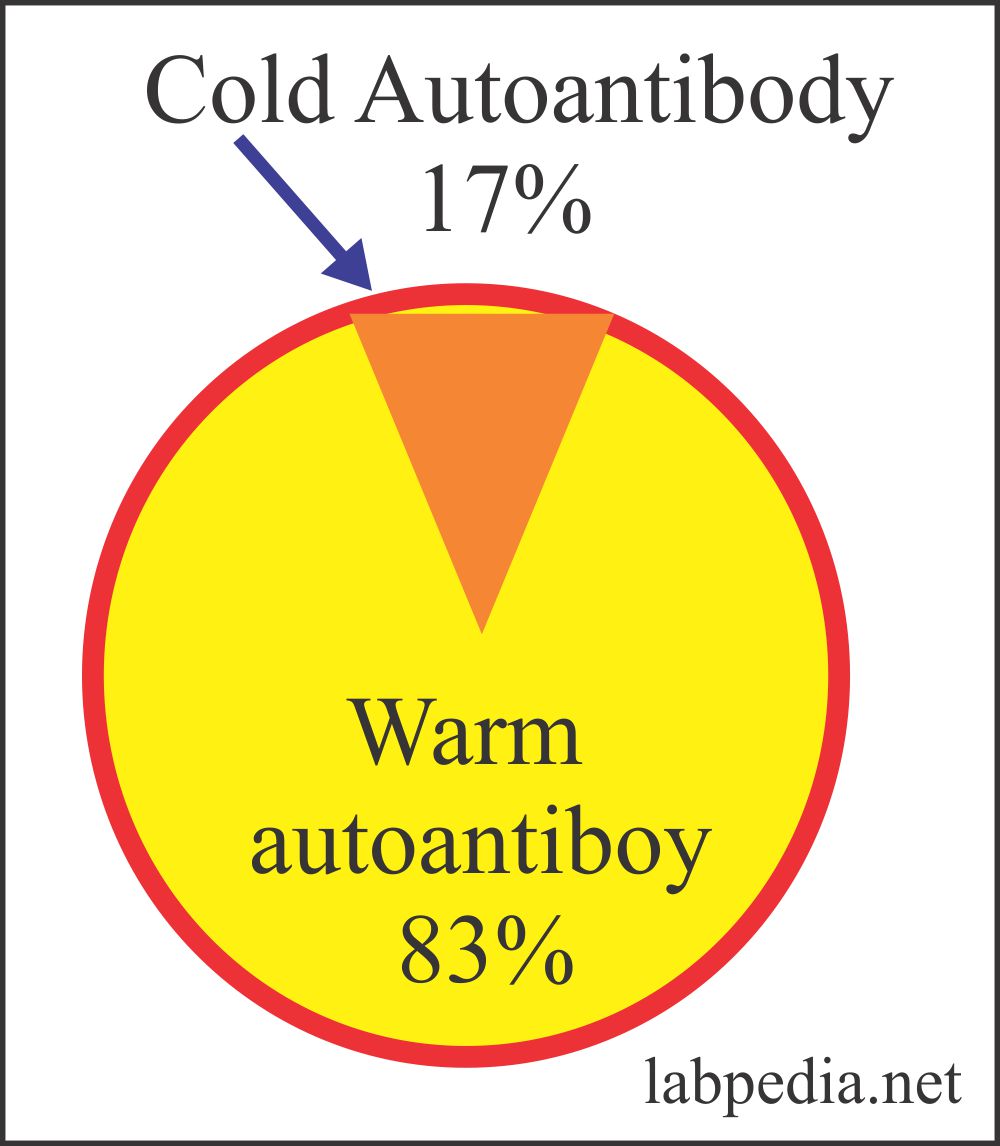
Autoimmune hemolytic anemia frequency:Type of autoimmune hemolytic anemia Frequency of the autoimmune hemolytic anemia Cold antibody AHA 16% Warm antibody AHA 70% Drug-induced AHA 12% Paroxysmal cold hemoglobinuria 1% to 2%
Classification of autoimmune hemolytic anemia:
| Type of anemia | Temperature of reaction |
TYpe of immunoglobulin (Ig) | Fixation and activation of complement | Causes (the type of diseases) |
Warm autoantibody AHA
|
37 °C | IgG (warm agglutinins) | Rare (limited) |
|
| Cod autoantibody AHA | 4 °C or below | IgM (cold agglutinin) | Frequently activates |
|
| Cold autoantibody AHA | 4 °C or below | IgG (cold hemolysin) | Always activates | Donath-Landsteiner antibody
|
Warm-antibody autoimmune hemolytic anemia:
- The autoantibodies are reactive at a warm temperature (37 °C) with the patient’s own RBCs, and Coomb’s direct test is positive. These antibodies will leads to warm autoimmune hemolytic anemia.
- Warm autoimmune hemolytic anemia may be:
- Idiopathic, without any underlying disease seen in 50% to 70% of the cases.
- Secondary, due to underlying pathology, is seen in 30% to 50% of the cases.
- In 3/4 cases, the RBCs are coated with antibody IgG and complement. Very rarely, IgA or IgM is seen.
- This coating may be only IgG or complement only.
- These are the most common type of autoimmune hemolytic anemia.
- Other conditions associated with warm antibody autoimmune hemolytic anemia are:
- Autoimmune diseases like:
- SLE.
- Rheumatoid arthritis.
- Pernicious anemia.
- Scleroderma.
- Infections are:
- Viral infections.
- Hepatitis B.
- Hepatitis A.
- Chronic diseases are:
- Chronic inflammatory diseases.
- Ulcerative colitis.
- Malignancies like:
- Ovarian tumors.
- Breast tumors.
- Lungs tumors.
- Kidneys tumors.
- Pancreatic tumors.
- Thymus tumors.
- Lymphoid neoplasia is:
- Hodgkin’s disease.
- Non-Hodgkin’s lymphoma.
- Waldenstrom’s macroglobulinemia.
- Multiple myeloma.
- Chronic lymphocytic leukemia.
- Autoimmune diseases like:
Clinical features:
- This AHA may occur at any age in either sex. This is slightly more common in females than in males.
- Most patients are above the age of 40 years.
- This anemia has variable severity.
- The patients will present with pallor, weakness, dizziness, and dyspnoea,
- The patient will have jaundice.
- These patients may develop a fever.
- It is splenomegaly.
- This anemia has remissions and relapses.
- This anemia may occur alone or in association with other diseases.
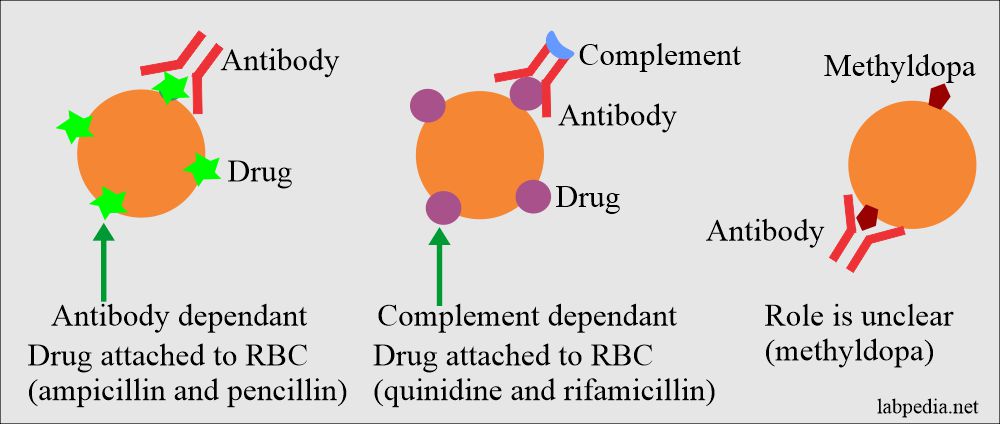
- This anemia may arise in patients taking methyldopa.
- Evans syndrome when this anemia appears along with idiopathic thrombocytopenia.
- In the case of SLE, the RBCs are coated with immunoglobulins and complement.
Mechanism of Hemolysis of warm autoantibody:
- The majority of 80-90% have RBC autoantibodies and reacts with their target RBC.
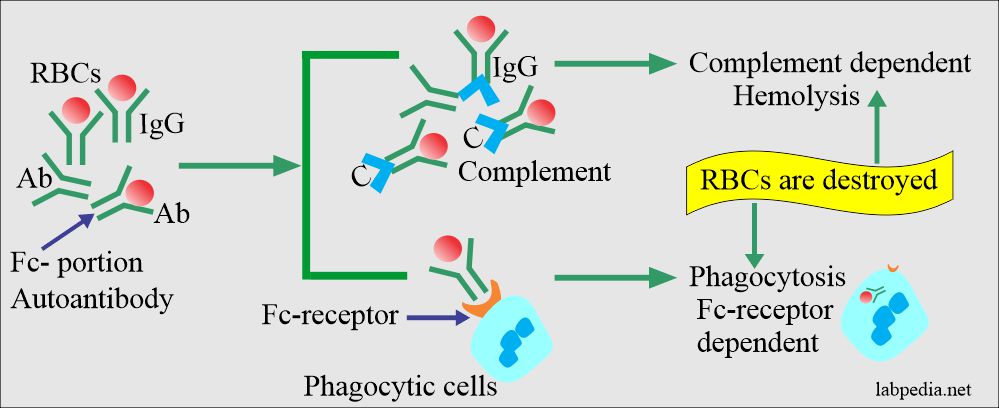
- Warm AHA: – The circulating RBC are coated with IgG. Complement is activated. Besides, components of complement are present on RBC. A warm autoantibody is mostly against Rh-antigen.
- Typically, these coated RBC are sequestered in the spleen and occasionally in the liver (extravascular hemolysis).
- The onset of autoimmune hemolysis is usually gradual in onset and may be precipitated by other factors like infection, trauma, psychological stress, and pregnancy.
- Autoantibodies produced in warm antibody autoimmune hemolytic anemia react with all cells tested.
- Laboratory findings:
- Coomb’s direct test is positive.
- Monospecific antiglobulin reagent gives direct Coomb’s test positive for IgG and C3d in 67% of the cases.
- The remaining cases are positive for IgG 20% or C3d 13% positive alone.
- In 80% of the cases, IgG alone, or with other immunoglobulins like IgM, and IgA or C3.
- All these findings are similar to extravascular hemolysis.
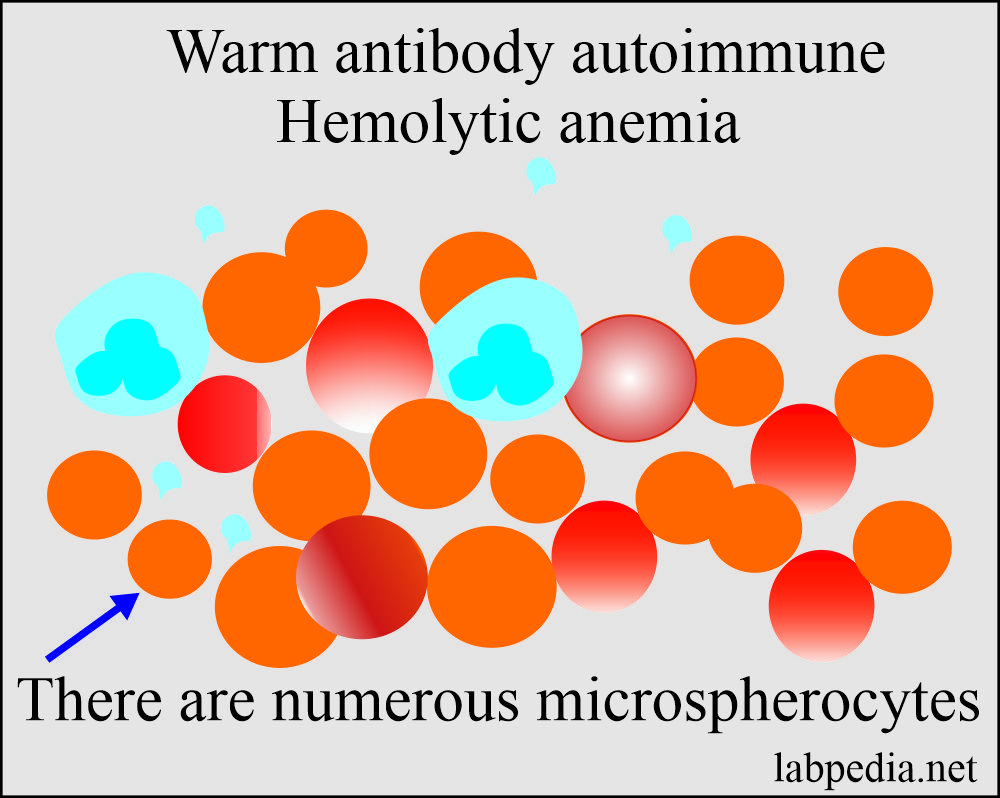
- Peripheral blood shows spherocytosis, and mostly are microspherocytes.
- There is moderate to severe normocytic, normochromic anemia.
- There are findings of extravascular hemolysis like polychromasia due to reticulocytosis.
- There are spherocytosis and red blood cell fragmentation.
- Rarely there may be nucleated RBC.
- Evidence of hemolysis will be seen like raised unconjugated bilirubin and urobilinogen.
- In severe hemolysis, haptoglobin will be decreased.
- Coomb’s test (direct antiglobulin test) is p[ositive.
- Antibodies both on the cell surface and in the serum are detected at 37 °C.
- Treatment:
- The best treatment is if you remove the cause of this AHA like methyldopa.
- Corticosteroids, like Prednisolone, are the first line of treatment, giving 60 mg/day, and later on, is tapered.
- Patients with only an antibody against RBCs responds well with steroids and splenectomy, while patients with complement respond poorly to both steroids and splenectomy.
- Spenemectomy is useful in most patients to maintain the hemoglobin level.
- Immunosuppression therapy may also be tried.
- Monoclonal antibodies can also be tried, mostly CD52 is tried.
- Folic acid is given in severe cases.
- Blood transfusion is also needed in severe anemia.
- High doses of immunoglobulin can be tried, but it has less success than ITP.
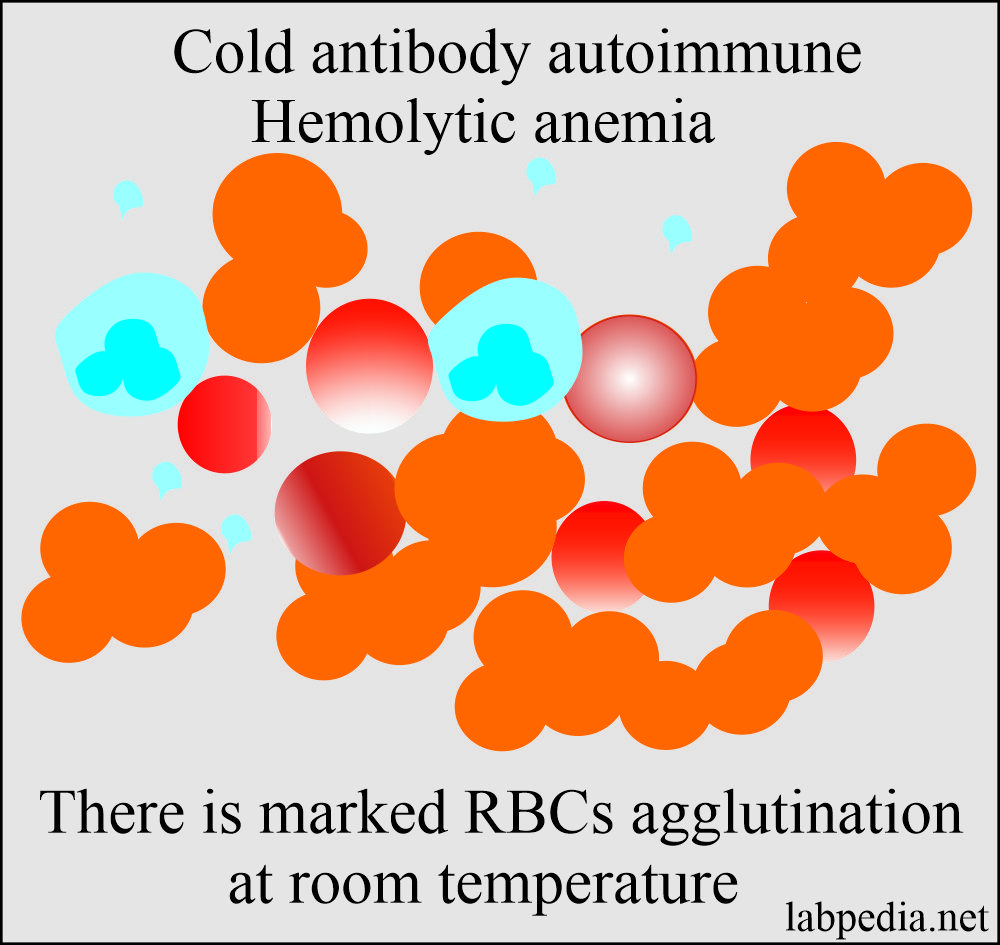
Cold-Antibody Autoimmune Hemolytic Anaemia:
- This may be acute or chronic form.
- The type of cold autoimmune hemolytic anemia:
- Idiopathic.
- Secondary:
- Monoclonal as seen in idiopathic cold hemagglutinins syndrome.
- Associated with lymphoproliferative disorders.
- Infections like, e.g., EBV, Mycoplasma Pneumoniae, and infectious mononucleosis. In these conditions, the antibody is attached to RBCs in the peripheral circulation.
- Paroxysmal cold hemoglobinuria.
- This may be:
- Idiopathic cold autoimmune hemolytic anemia or called cold agglutinin syndrome.
- The chronic form is found mostly in the older adults complaining of Raynaud’s phenomenon.
- The common antibodies are IgM, which causes agglutination in the extremities and fixing of complement, leading to ultimately intravascular hemolysis.
- The secondary type is found in 50% of the cases due to Mycoplasma pneumonia. This will resolve within 2 to 3 weeks.
- Idiopathic cold autoimmune hemolytic anemia or called cold agglutinin syndrome.
- These cold reacting auto-antibody has a strong reaction at 4 °C but may react up to 22 °C.
- The titer of benign cold autoantibody is usually <64 at 4 °C, while the pathologic cold autoantibodies titer is >1000 at 4 °C.
- Mostly there is IgM autoantibody and can activate Complement in vitro.
- The acute form is due to Mycoplasma pneumonia infection or lymphoproliferative disorders like lymphomas.
- The chronic form is seen in older people and produces mild to moderate type of hemolysis.
- Raynaud’s phenomenon and hemoglobinuria are seen in cold weather.
- Benign cold autoantibodies are:
- There is normal cold autoagglutinins circulation in the blood circulation.
- These agglutinins are very weak and give no clinical signs and symptoms.
- The examples are blood group I, H, and IH. The cold antibodies anti-I, anti-H, and anti-IH do not give significant symptoms, and these antibodies are so weak that the serological test can not detect these.
- These antibodies have a very low concentration in the blood.
- Mechanism of hemolysis in cold autoantibody:
- Pathologic cold autoantibodies are divided into two groups:
- Primary or idiopathic gives rise to cold agglutinin syndrome.
- Secondary cold agglutinins are due to infections and are called secondary cold agglutinins syndrome.
- The third type is seen in paroxysmal cold hemoglobinuria.
- IgM’s cold autoimmune antibodies attach to the surface of the RBCs at 4 °C.
- These antibodies react with the RBCs when the body temperature falls below 32 °C or below.
- The complement system activation is efficient, is activated, and leads to hemolysis, extravascular, or intravascular.
- Pathologic cold autoantibodies are divided into two groups:
- Clinical signs and symptoms:
- These patients have chronic hemolytic anemia aggravated by the cold and often associated with intravascular hemolysis.
- There are mild splenomegaly and jaundice.
- The patient may develop acrocyanosis (purplish skin discoloration) particularly prominent on the tip of the nose, ears, fingers, and toes.
- Agglutination of the RBCs is seen in the small blood vessels.
- Laboratory findings:
- Peripheral blood shows similar changes like warm antibody anemia, except there are no prominent spherocytes.
- Direct anticoagulant test (Coomb’s test) shows complement (C3d) only the RBCs.
- Treatment: Keep the patients warm and treating the underlying cause.
- Chemotherapy, like chlorambucil, is helpful in chronic cases. Anti-CD20 (rituximab) and CD52 (Campath-IH) have been used.
- Splenectomy is not helpful unless there is massive splenomegaly.
- Exclude the idiopathic cases.
Paroxysmal cold hemoglobinuria (PCH):
- This is rare, and this is the least type of autoimmune hemolytic anemia seen in 1% to 2%.
- The Donath-Landsteiner antibody causes an IgG antibody with specificity for the P blood group antigen, which binds to the RBCs in the cold but causes hemolysis in the warm condition.
- This was originally described in patients with syphilis in the old days. Now it is frequently seen in acute transient conditions:
- Viral diseases, particularly in children like measles, mumps, chickenpox, and infectious mononucleosis.
- Idiopathic diseases in older patients.
- Clinical findings:
- These patients with acute attack suddenly develop when exposed to cold:
- Fever.
- Chills.
- Abdominal and back pain.
- There is jaundice.
- There is hemoglobinuria,
- The resultant anemia may be severe.
- These patients with acute attack suddenly develop when exposed to cold:
- Mechanism:
- This is an IgG type of antibody that reacts with RBCs in colder parts of the body, like feet and hands.
- There is the activation of the complement system and the emergence of C3 and C4.
- C3 and C4 will bind the RBCs irreversibly.
- At warmer temperatures, the RBCs are hemolysed.
- Treatment:
- This is keeping the patient warm, and, if needed, a blood transfusion may be given.
- Syphilis and viral infections are usually self-limiting diseases.
Drug-Induced Hemolysis (Drug-induced hemolytic anemia):
Drugs induced hemolysis may lead to hemolytic anemia.
There is a coating of the RBCs with the drugs, and it is shown by Coomb’s direct test positive, and this drug leads to hemolysis.
Example are:
- Penicillin is the prototype.
- 3% of the patients on penicillin therapy will be Coomb’s direct test positive.
- 5% of these Coomb’s test positive cases will have hemolysis.
- Methyldopa autoimmune hemolytic anemia. This is the most common drug-induced hemolytic anemia accounting for roughly 70% of all the cases.
- The antibodies produced by this drug are true autoantibodies.
- Drugs producing hemolysis like methyldopa are α-methyldopa, (Aldomet), L-dopa, procainamide, chlorpromazine, ibuprofen, ceftriaxone, and mefenamic acid.
- Drugs that lead to absorption mechanisms are penicillin, diclofenac, and cephalosporin.
- Drugs that form the immune-complex are antihistamine, isoniazid, rifampicin, streptomycin, sulfonamides, tetracyclines, acetaminophen, and chlorpromazine.
- Drugs that give rise to membrane modification mechanism is a cephalosporin.
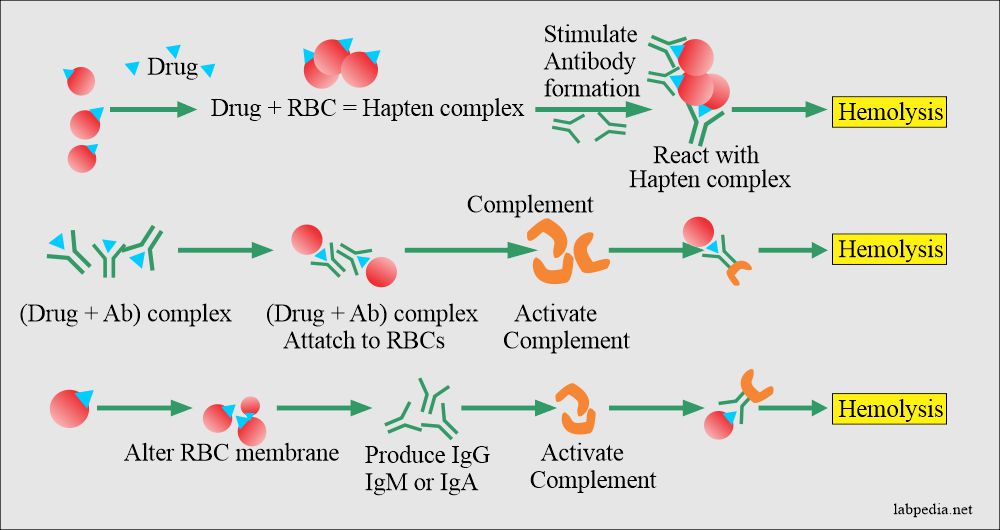
Mechanism:-
The basic mechanism of hemolysis are:
- Drug absorption.
- Immune complex formation.
- There is a modification of the cell membrane.
- There is autoantibodies formation.
- Autoantibody formation: The most common cause of hemolytic anemia is the drug α-methyldopa, where IgG-type autoantibody is formed against the Rh-antigens.
- There will be a bypass of Th cell, and B-L is activated and gives rise to anti-Rh Ab.
- Hapten-complex formation: The drug attaches to RBC surface antigens, making them immunogenic (drug+RBC) hapten complex.
- This haptens complex form antibody, which will react with the (drug+RBC) hapten complex.
- Membrane modification: There may be membrane modification where the drugs attach to the RBC cell membrane.
- Drug absorption: This will alter the cell (RBC) membrane, and there is nonspecific absorption of IgG, IgM, or IgA to RBC, e.g., penicillin.
- Also, there is an attachment of the complement and activation leading to hemolysis.
- In this method, the drugs act as haptens like penicillin and cyclosporins.
- These drugs attach to a protein on the RBC. Now, this becomes an immunogen and cause immune damage.
- This mechanism is seen with the drug-quinidine (hapten), which binds to plasma protein to become immunogenic. This complex is absorbed on to RBC.
- Autoantibody formation: The most common cause of hemolytic anemia is the drug α-methyldopa, where IgG-type autoantibody is formed against the Rh-antigens.
Various methods by which drugs can cause hemolysis of the red blood cells:
Comparison of warm autoantibody and cold autoantibody autoimmune hemolytic anemia:
| Characteristic differentiating points | Cold autoantibody hemolytic anemia | Warm autoantibody hemolytic anemia |
| Frequency | 16% of the cases and PCH is 1% to 2% | 70% to 75% of the cases |
| Hemolysis |
|
|
| Temperature for the activity | <30 °C ( optimal temperature is 4 °C to 22 °C) | >32 °C |
| Type of immunoglobulins |
|
IgG |
| Role of the complement | Binds and activated | May binds and activate |
| The specificity of the reaction | PCH is anti-P | Frequently Rh |
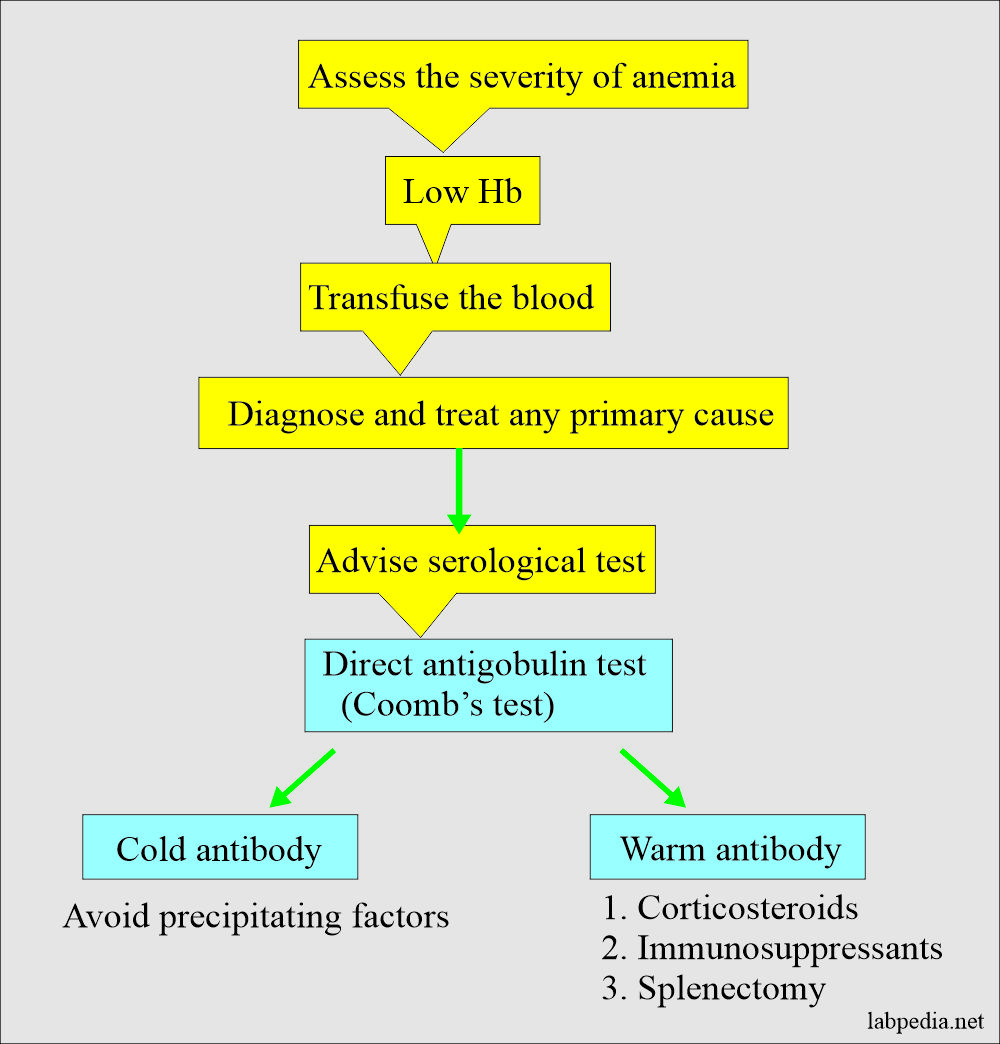
Management
Management of Autoimmune hemolytic Anaemia can be summarized as follows.
Diagnosis of Autoimmune hemolytic anemia:
- The peripheral blood smear shows spherocytes.
- Coomb’s test is positive.
- Temperature-dependent agglutination reactions will help to identify the specific type of autoantibody.
- Keep warm the patients.
- Corticosteroids are given.